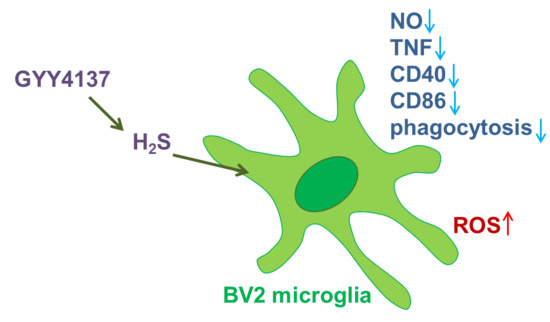
The H2S Donor GYY4137 Stimulates Reactive Oxygen Species Generation in BV2 Cells While Suppressing the Secretion of TNF and Nitric Oxide
Abstract
:1. Introduction
2. Results
2.1. GYY4137 Inhibits Production of NO and TNF in BV2 Cells
2.2. GYY4137 Influences BV2 Cell Phenotype and Phagocytic Ability
2.3. GYY4137 Upregulates ROS Generation in BV2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Reagents
4.2. Viability Assays
4.3. Detection of NO Release
4.4. ELISA Test for Determination of Cytokines
4.5. Real-Time Reverse Transcription Polymerase Chain Reaction
4.6. Cytofluorimetry
4.7. Detection of Reactive Oxygen Species (ROS) Generation
4.8. Real-Time Cell Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Wallace, J.L.; Blackler, R.W.; Chan, M.V.; Da Silva, G.J.; Elsheikh, W.; Flannigan, K.L.; Gamaniek, I.; Manko, A.; Wang, L.; Motta, J.-P.; et al. Anti-inflammatory and cytoprotective actions of hydrogen sulfide: translation to therapeutics. Antioxid. Redox Signal. 2015, 22, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mazzon, E.; Bramanti, P.; Bendtzen, K.; Nicoletti, F. Gasotransmitters and the immune system: Mode of action and novel therapeutic targets. Eur. J. Pharmacol. 2018, 834, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.-H.; Shi, X.-R.; Hong, Z.-Y.; Pan, L.-L.; Liu, X.-H.; Zhu, Y.-Z. A new hope for neurodegeneration: possible role of hydrogen sulfide. J. Alzheimers Dis. 2011, 24 (Suppl. 2), 173–182. [Google Scholar] [CrossRef]
- Xue, X.; Bian, J.-S. Neuroprotective effects of hydrogen sulfide in Parkinson’s disease animal models: methods and protocols. Methods Enzymol. 2015, 554, 169–186. [Google Scholar] [PubMed]
- Talaei, F. Pathophysiological Concepts in Multiple Sclerosis and the Therapeutic Effects of Hydrogen Sulfide. Basic Clin. Neurosci. 2016, 7, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Adamska, P.; Bentke, A.; Wróbel, M. Hydrogen sulfide generation from l-cysteine in the human glioblastoma-astrocytoma U-87 MG and neuroblastoma SHSY5Y cell lines. Acta Biochim. Pol. 2017, 64, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, Y.; Wang, Z.; Kong, Y.; Gao, R.; Chen, G. Hydrogen sulfide therapy in brain diseases: from bench to bedside. Med. Gas Res. 2017, 7, 113. [Google Scholar] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, Y.; Chen, Y.; Zhu, J.; Yang, Y.; Zhang, H.-L. Role of Microglia in Neurological Disorders and Their Potentials as a Therapeutic Target. Mol. Neurobiol. 2017, 54, 7567–7584. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.; Madore, C.; Lassmann, H.; Butovsky, O. Microglial Phenotypes and Functions in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.; Mazzon, E.; Basile, M.S.; Di Marco, R.; Bramanti, P.; Mammana, S.; Petralia, M.C.; Fagone, P.; Nicoletti, F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget 2018, 9, 17951–17970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presti, M.; Mazzon, E.; Basile, M.S.; Petralia, M.C.; Bramanti, A.; Colletti, G.; Bramanti, P.; Nicoletti, F.; Fagone, P. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol. Lett. 2018, 16, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.; McDonald, K.; Grewal, T.; Munoz, L. Interleukins in glioblastoma pathophysiology: implications for therapy. Br. J. Pharmacol. 2013, 168, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Sugama, S.; Takenouchi, T.; Cho, B.P.; Joh, T.H.; Hashimoto, M.; Kitani, H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm. Allergy Drug Targets 2009, 8, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zindler, E.; Zipp, F. Neuronal injury in chronic CNS inflammation. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.D.; Shriver, L.P.; Maresz, K.; Dittel, B.N. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005, 81, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, Y.; Ao, G.; Hu, L.; Liu, H.; Wu, J.; Wang, X.; Jin, M.; Zheng, S.; Zhen, X.; et al. CaMKKβ-Dependent Activation of AMP-Activated Protein Kinase Is Critical to Suppressive Effects of Hydrogen Sulfide on Neuroinflammation. Antioxid. Redox Signal. 2014, 21, 1741–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.-H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010, 12, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, L.; Ang, A.D.; Zhang, H.; Moore, P.K.; Bhatia, M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J. Leukoc. Biol. 2007, 81, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Liao, X.; Mo, L.; Yang, C.; Yang, Z.; Wang, X.; Hu, F.; Chen, P.; Feng, J.; Zheng, D.; et al. Hydrogen sulfide protects against chemical hypoxia-induced injury by inhibiting ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells. PLoS ONE 2011, 6, e25921. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-B.; Yang, C.-T.; Zheng, D.-D.; Mo, L.-Q.; Wang, X.-Y.; Lan, A.-P.; Hu, F.; Chen, P.-X.; Feng, J.-Q.; Zhang, M.-F.; et al. Inhibition of ROS-activated ERK1/2 pathway contributes to the protection of H2S against chemical hypoxia-induced injury in H9c2 cells. Mol. Cell. Biochem. 2012, 362, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, X.; Zhao, S.; Wei, C.; Yin, Y.; Liu, T.; Jiang, S.; Xie, J.; Wan, X.; Mao, M.; et al. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem. Int. 2013, 63, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sandhir, R. Neuroprotective Effect of Hydrogen Sulfide in Hyperhomocysteinemia Is Mediated Through Antioxidant Action Involving Nrf2. Neuromol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, M.A.; Pennefather, P.S.; O’Brien, P.J. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 2004, 203, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Wagner, E.D.; Gaskins, H.R.; Plewa, M.J. Hydrogen Sulfide Induces Direct Radical-Associated DNA Damage. Mol. Cancer Res. 2007, 5, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyner-Matos, J.; Predmore, B.L.; Stein, J.R.; Leeuwenburgh, C.; Julian, D. Hydrogen Sulfide Induces Oxidative Damage to RNA and DNA in a Sulfide-Tolerant Marine Invertebrate. Physiol. Biochem. Zool. 2010, 83, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Scuffi, D.; Nietzel, T.; Di Fino, L.M.; Meyer, A.J.; Lamattina, L.; Schwarzländer, M.; Laxalt, A.M.; García-Mata, C. Hydrogen Sulfide Increases Production of NADPH Oxidase-Dependent Hydrogen Peroxide and Phospholipase D-Derived Phosphatidic Acid in Guard Cell Signaling. Plant Physiol. 2018, 176, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-H.; Wei, Z.-Z.; Hu, K.-D.; Hu, L.-Y.; Li, Y.-H.; Chen, X.-Y.; Han, Z.; Yao, G.-F.; Zhang, H. Hydrogen sulfide inhibits the growth of Escherichia coli through oxidative damage. J. Microbiol. 2018, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-L.; Wan, X.-H.; Chen, Y.; Bi, C.; Chen, H.-M.; Zhong, Y.; Heng, X.-H.; Qian, J.-Q. H2S Protects Hippocampal Neurons from Anoxia–Reoxygenation Through cAMP-Mediated PI3K/Akt/p70S6K Cell-Survival Signaling Pathways. J. Mol. Neurosci. 2011, 43, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.-J.; He, L.; Zhang, W.; Liu, C.-L.; Ai, Y.-Q.; Zhang, Q. Hydrogen sulfide attenuates surgical trauma-induced inflammatory response and cognitive deficits in mice. J. Surg. Res. 2013, 183, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, Y.; Lin, W.; Gao, D.; Fei, Z. Protective effects of hydrogen sulfide in a rat model of traumatic brain injury via activation of mitochondrial adenosine triphosphate–sensitive potassium channels and reduction of oxidative stress. J. Surg. Res. 2013, 184, e27–e35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, L.; Liu, D.; Wang, J.; Wang, S.; Zhang, Q.; Gong, Y.; Liu, H.; Hao, A.; Wang, Z. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol. Res. 2014, 84, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lu, Z.; Tao, L.; Yang, L.; Guo, Y.; Yang, Y.; Sun, X.; Ding, Q. ROS-Dependent Neuroprotective Effects of NaHS in Ischemia Brain Injury Involves the PARP/AIF Pathway. Cell. Physiol. Biochem. 2015, 36, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013, 104, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yuan, Y.; Sheng, Y.; Yuan, B.; Wang, Y.; Zheng, J.; Liu, C.-F.; Zhang, X.; Hu, L.-F. GYY4137, an H2S Slow-Releasing Donor, Prevents Nitrative Stress and α-Synuclein Nitration in an MPTP Mouse Model of Parkinson’s Disease. Front. Pharmacol. 2017, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-F.; Zhang, D.-D.; Yu, X.-J.; Gao, H.-L.; Liu, K.-L.; Qi, J.; Li, H.-B.; Yi, Q.-Y.; Chen, W.-S.; Cui, W.; et al. Hydrogen sulfide in paraventricular nucleus attenuates blood pressure by regulating oxidative stress and inflammatory cytokines in high salt-induced hypertension. Toxicol. Lett. 2017, 270, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Xu, Z.; Xiao, B.; Han, Z.; Huang, J.; Xu, J.; Lun, Z.; Zhou, W. Hydrogen sulfide protects against the development of experimental cerebral malaria in a C57BL/6 mouse model. Mol. Med. Rep. 2017, 16, 2045–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Shan, H.; Chang, P.; Wang, T.; Dong, W.; Chen, X.; Tao, L. Hydrogen Sulfide Offers Neuroprotection on Traumatic Brain Injury in Parallel with Reduced Apoptosis and Autophagy in Mice. PLoS ONE 2014, 9, e87241. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Zhou, X.; Li, H.; Yang, X.; Dong, Z.; Zhou, W.; Chen, J. Hydrogen Sulfide Promotes Learning and Memory and Suppresses Proinflammatory Cytokines in Repetitive Febrile Seizures. Neuroimmunomodulation 2016, 23, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, X.; Xu, Y.; He, M.; Yang, J.; Li, J.; Li, Y.; Ao, G.; Cheng, J.; Jia, J. The cystathionine β-synthase/hydrogen sulfide pathway contributes to microglia-mediated neuroinflammation following cerebral ischemia. Brain. Behav. Immun. 2017, 66, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Chen, C.P.L.H.; Halliwell, B.; Moore, P.K.; Wong, P.T.-H. Hydrogen Sulfide Is a Mediator of Cerebral Ischemic Damage. Stroke 2006, 37, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juurlink, B.H.; Sweeney, M.I. Mechanisms that result in damage during and following cerebral ischemia. Neurosci. Biobehav. Rev. 1997, 21, 121–128. [Google Scholar] [CrossRef]
- Wong, P.T.H.; Qu, K.; Chimon, G.N.; Seah, A.B.H.; Chang, H.M.; Wong, M.C.; Ng, Y.K.; Rumpel, H.; Halliwell, B.; Chen, C.P.L.H. High Plasma Cyst(e)ine Level May Indicate Poor Clinical Outcome in Patients With Acute Stroke: Possible Involvement of Hydrogen Sulfide. J. Neuropathol. Exp. Neurol. 2006, 65, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longen, S.; Beck, K.-F.; Pfeilschifter, J. H2S-induced thiol-based redox switches: Biochemistry and functional relevance for inflammatory diseases. Pharmacol. Res. 2016, 111, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Créange, A.; Orlikowski, D.; Bolgert, F.; Mangano, K.; Metz, C.; Di Marco, R.; Al Abed, Y. Macrophage migration inhibitory factor (MIF) seems crucially involved in Guillain-Barré syndrome and experimental allergic neuritis. J. Neuroimmunol. 2005, 168, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mazzon, E.; Cavalli, E.; Bramanti, A.; Petralia, M.C.; Mangano, K.; Al-Abed, Y.; Bramati, P.; Nicoletti, F. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: In silico and in vivo evidences. J. Neuroimmunol. 2018, 322, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, P.; Fehérvári, Z.; Blanchard, L.; Nicoletti, F.; Edwards, C.K., III; Cooke, A. Autoimmune thyroid disease induced by thyroglobulin and lipopolysaccharide is inhibited by soluble TNF receptor type I. Eur. J. Immunol. 2002, 32, 1021–1028. [Google Scholar] [CrossRef]
- Peng, F.; Wang, X.; Shu, M.; Yang, M.; Wang, L.; Ouyang, Z.; Shen, C.; Hou, X.; Zhao, B.; Wang, X.; et al. Raddeanin a Suppresses Glioblastoma Growth by Inducing ROS Generation and Subsequent JNK Activation to Promote Cell Apoptosis. Cell. Physiol. Biochem. 2018, 47, 1108–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratton, R.; Tricarico, P.M.; Guimaraes, R.L.; Celsi, F.; Crovella, S. Lopinavir/Ritonavir Treatment Induces Oxidative Stress and Caspaseindependent Apoptosis in Human Glioblastoma U-87 MG Cell Line. Curr. HIV Res. 2018, 16, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic-Ivanic, D.; Fagone, P.; McCubrey, J.; Bendtzen, K.; Mijatovic, S.; Nicoletti, F. HIV-protease inhibitors for the treatment of cancer: Repositioning HIV protease inhibitors while developing more potent NO-hybridized derivatives? Int. J. Cancer 2017, 140, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-T.; Chen, L.; Xu, S.; Day, J.J.; Li, X.; Xian, M. Recent Development of Hydrogen Sulfide Releasing/Stimulating Reagents and Their Potential Applications in Cancer and Glycometabolic Disorders. Front. Pharmacol. 2017, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Gemici, B.; Elsheikh, W.; Feitosa, K.B.; Costa, S.K.P.; Muscara, M.N.; Wallace, J.L. H2S-releasing drugs: Anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide 2015, 46, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vaughan, D.; Dicay, M.; MacNaughton, W.K.; de Nucci, G. Hydrogen Sulfide-Releasing Therapeutics: Translation to the Clinic. Antioxid. Redox Signal. 2018, 28, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević, M.; Mazzon, E.; Momčilović, M.; Basile, M.S.; Colletti, G.; Petralia, M.C.; Bramanti, P.; Nicoletti, F.; Miljković, Đ. The H2S Donor GYY4137 Stimulates Reactive Oxygen Species Generation in BV2 Cells While Suppressing the Secretion of TNF and Nitric Oxide. Molecules 2018, 23, 2966. https://doi.org/10.3390/molecules23112966
Lazarević M, Mazzon E, Momčilović M, Basile MS, Colletti G, Petralia MC, Bramanti P, Nicoletti F, Miljković Đ. The H2S Donor GYY4137 Stimulates Reactive Oxygen Species Generation in BV2 Cells While Suppressing the Secretion of TNF and Nitric Oxide. Molecules. 2018; 23(11):2966. https://doi.org/10.3390/molecules23112966
Chicago/Turabian StyleLazarević, Milica, Emanuela Mazzon, Miljana Momčilović, Maria Sofia Basile, Giuseppe Colletti, Maria Cristina Petralia, Placido Bramanti, Ferdinando Nicoletti, and Đorđe Miljković. 2018. "The H2S Donor GYY4137 Stimulates Reactive Oxygen Species Generation in BV2 Cells While Suppressing the Secretion of TNF and Nitric Oxide" Molecules 23, no. 11: 2966. https://doi.org/10.3390/molecules23112966