Biological Activity of the Carrier as a Factor in Immunogen Design for Haptens
Abstract
:1. Introduction
2. Results
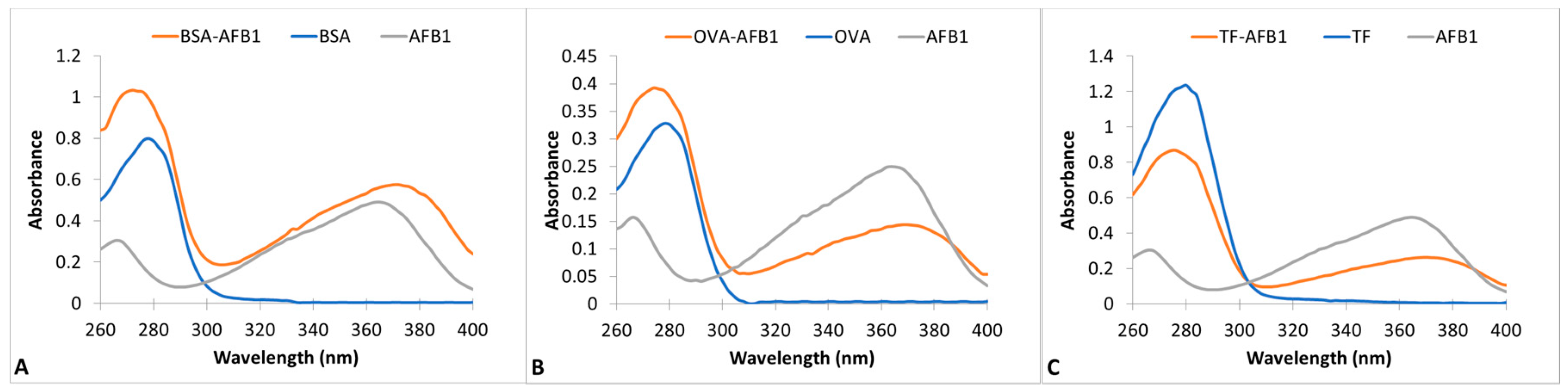
2.1. Preparation and Characterization of AF–Protein Conjugates
2.2. Immune Response
2.3. Monoclonal Antibody Development
3. Discussion
4. Materials and Methods
4.1. Materials and Equipment
4.2. Preparation of AF–Protein Conjugates
4.3. Immunizations
4.4. Indirect ELISA
4.5. Indirect Competitive ELISA
4.6. Development of Monoclonal Antibodies
4.7. Ethical Considerations
4.8. Safety Considerations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yolaç, E.; Pınarbaşı, A.; Ertekin, Ö.; Güloğlu, F.B.; Öztürk, S. Improving the approaches to increase the immunogenicity of Staphylococcal enteretoksin B. New Biotechnol. 2012, 29, S223. [Google Scholar] [CrossRef]
- Regenmortel, M. Van Antigenicity and immunogenicity of synthetic peptides. Biologicals 2001, 29, 209–2013. [Google Scholar] [CrossRef] [PubMed]
- Crumpton, M. Protein antigens: The molecular bases of antigenicity and immunogenicity. Antigens 1974, 2, 1–78. [Google Scholar]
- Murphy, K. Garland Science. In Janeway’s Immunobiology, 8th ed.; Garland Science: New York, NY, USA, 2011. [Google Scholar]
- Ertekin, Ö.; Pirinçci, Ş.; Öztürk, S. Monoclonal IgA Antibodies for Aflatoxin Immunoassays. Toxins 2016, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Caro-Maldonado, A.; Wang, R.; Nichols, A.G.; Kuraoka, M.; Milasta, S.; Sun, L.D.; Gavin, A.L.; Abel, E.D.; Kelsoe, G.; Green, D.R.; et al. Metabolic Reprogramming Is Required for Antibody Production That Is Suppressed in Anergic but Exaggerated in Chronically BAFF-Exposed B Cells. J. Immunol. 2014, 192, 3626–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertekin, Ö.; Öztürk, S.; Öztürk, Z.Z. Label Free QCM Immunobiosensor for AFLB1 Detection Using Monoclonal IgA Antibody as Recognition Element. Sensors 2016, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Pirinçci, Ş.Ş.; Ertekin, Ö.; Laguna, D.E.; Özen, F.Ş.; Öztürk, Z.Z.; Öztürk, S. Label-free QCM immunosensor for the detection of ochratoxin A. Sensors 2018, 18, 1161. [Google Scholar] [CrossRef] [PubMed]
- Boothby, M.; Rickert, R.C. Metabolic Regulation of the Immune Humoral Response. Immunity 2017, 46, 743–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertekin, Ö.; Pirinçci, Ş.Ş.; Öztürk, S. Monoclonal Antibodies MAM-D3E4, MAM-D3C6, MAM-D12E2, and MAM-D4D6 Against Aflatoxins. Monoclon. Antib. Immunodiagn. Immunother. 2016, 35, 117–118. [Google Scholar]
- Ertekin, Ö.; Arslankaraoğlu Akçael, E.; Yücel, F. Monoclonal Antibodies MAM-2B11, MAM-6E10, and MAM-8G12 Against Aflatoxins. Monoclon. Antib. Immunodiagn. Immunother. 2016, 35, 119–120. [Google Scholar]
- Hwang, C.S.; Smith, L.C.; Natori, Y.; Ellis, B.; Zhou, B.; Janda, K.D. Improved Admixture Vaccine of Fentanyl and Heroin Hapten Immunoconjugates: Antinociceptive Evaluation of Fentanyl-Contaminated Heroin. ACS Omega 2018, 3, 11537–11543. [Google Scholar] [CrossRef] [PubMed]
- Bremer, P.T.; Schlosburg, J.E.; Banks, M.L.; Steele, F.F.; Zhou, B.; Poklis, J.L.; Janda, K.D. Development of a Clinically Viable Heroin Vaccine. J. Am. Chem. Soc. 2017, 139, 8601–8611. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Wally, J.; Halbrooks, P.J.; Vonrhein, C.; Rould, M.A.; Everse, S.J.; Mason, A.B.; Buchanan, S.K. The crystal structure of iron-free human serum transferrin provides insight into inter-lobe communication and receptor binding. J. Biol. Chem. 2006, 281, 24934–24944. [Google Scholar] [CrossRef] [PubMed]
- Kleven, M.D.; Jue, S.; Enns, C.A. Transferrin Receptors TfR1 and TfR2 Bind Transferrin through Differing Mechanisms. Biochemistry 2018, 57, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, S.; Çirakoglu, B.; Bermek, E. Partial Characterization of the Human Serum Transferrin Epitope Reactive with the Monoclonal Antibody TRC-2. Hybrid. Hybridomics 2003, 22, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Helguera, G.; Penichet, M.L. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin. Immunol. 2006, 121, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Engler, J.A.; Collawn, J.F.; Moore, B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem. 2001, 268, 2004–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilner, S.E.; Wengerter, B.; Maier, K.; de Lourdes Borba Magalhães, M.; Del Amo, D.S.; Pai, S.; Opazo, F.; Rizzoli, S.O.; Yan, A.; Levy, M. An RNA Alternative to Human Transferrin: A New Tool for Targeting Human Cells. Mol. Ther. Nucleic Acids 2012, 1, e21. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S. Aflatoxin analysis at the beginning of the twenty-first century. Anal. Bioanal. Chem. 2009, 395, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zak, O.; Aisen, P.; Harrison, S.C.; Walz, T. Structure of the human transferrin receptor-transferrin complex. Cell 2004, 116, 565–576. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Yu, W.; Xu, Y.; Wang, P.; Xie, B.; Chen, F. Preparation for aflatoxin B1-cationized bovine serum albumin based on Mannich-type reaction. J. Immunol. Methods 2007, 328, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Patricia, L.; Domen, G.H. Cationized Carriers for Immunogen Production. U.S. Patent 5,142,027, 25 August 1992. [Google Scholar]
- Michael, J.G. Cationization of protein antigens. VI. Effects of cationization on the immunoregulatory properties of a bovine serum albumin peptide, a.a. 506–589. Cell. Immunol. 1991, 138, 121–129. [Google Scholar] [CrossRef]
- Xu, Q.H.; Zhao, X.N.; Cheng, J.P.; Wei, C.H.; Zhang, Q.H.; Rong, K.T. Influence of carrier proteins on the immunologic response to haptenic antitetrodotoxin vaccine. Bioconjug. Chem. 2006, 17, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Laptev, R.; Nisnevitch, M.; Siboni, G.; Malik, Z.; Firer, M.A. Intracellular chemiluminescence activates targeted photodynamic destruction of leukaemic cells. Br. J. Cancer 2006, 95, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.A.; Joao, H.C.; Hammerschmid, F.; Eder, J.; Steinkasserer, A. An antigenic HIV-1 peptide sequence engineered into the surface structure of transferrin does not elicit an antibody response. FEBS Lett. 1999, 459, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-P.; Deng, Y.-J.; Jin, X.-Y.; Chen, L.-G.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Ultrasensitive electrochemical immunosensor for ochratoxin A using gold colloid-mediated hapten immobilization. Anal. Biochem. 2009, 389, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Srivastava, S.; Raghava, G.P.S.; Varshney, G.C. HaptenDB: A comprehensive database of haptens, carrier proteins and anti-hapten antibodies. Bioinformatics 2006, 22, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Gefen, T.; Vaya, J.; Khatib, S.; Rapoport, I.; Lupo, M.; Barnea, E.; Admon, A.; Heller, E.D.; Aizenshtein, E.; Pitcovski, J. The effect of haptens on protein-carrier immunogenicity. Immunology 2015, 144, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Samsonova, J.V.; Baxter, G.A.; Crooks, S.R.H.; Small, A.E.; Elliott, C.T. Determination of ivermectin in bovine liver by optical immunobiosensor. Biosens. Bioelectron. 2002, 17, 523–529. [Google Scholar] [CrossRef]
- Crooks, S.R.H.; Baxter, A.G.; McCaughey, W.J.; Rabel, S.R.; Stobaugh, J.F.; Heinig, R.; Bostick, J.M.; Tway, P.C.; Wood, J.S.; Downing, G.V.; et al. Detection of ivermectin residues in bovine liver using an enzyme immunoassay. Analyst 1998, 123, 355–358. [Google Scholar] [CrossRef]
- Crowther, J.R. The ELISA Guidebook, 2nd ed.; Walker, J.M., Ed.; Springer: Hertfordshire, UK, 2002. [Google Scholar]
- Engvall, E.; Jonsson, K.; Perlmann, P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim. Biophys. Acta 1971, 251, 427–434. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, H.; Fan, G.; Ma, J.; Wang, Z.; Wang, J. Preparation of monoclonal antibody based indirect competitive ELISA for detecting 19-nortestosterone residue. Chin. Sci. Bull. 2011, 56, 2698–2705. [Google Scholar] [CrossRef] [Green Version]
- Köhler, G. Fusion of Lymphocytes. Immunol. Method 1979, 391–395. [Google Scholar]
- Yücel, F.; Ertekin, Ö. Hybridoma technology. In Current Applications in Biotechnology; Dündar, M., Bağış, H., Eds.; Erciyes University publications: Kayseri, Turkey, 2017; pp. 189–210. [Google Scholar]
- Greenfield, E.A. Antibodies: A Laboratory Manual, 2nd ed.; Greenfield, A.E., Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2014. [Google Scholar]
- Maher, A. Monoclonal Antibodies. In Methods in Molecular Biology; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2007; Volume 378. [Google Scholar]
Sample Availability: Samples of the compounds: BSA-AFB1, OVA-AFB1 and TF-AFB1 are available from the authors. |

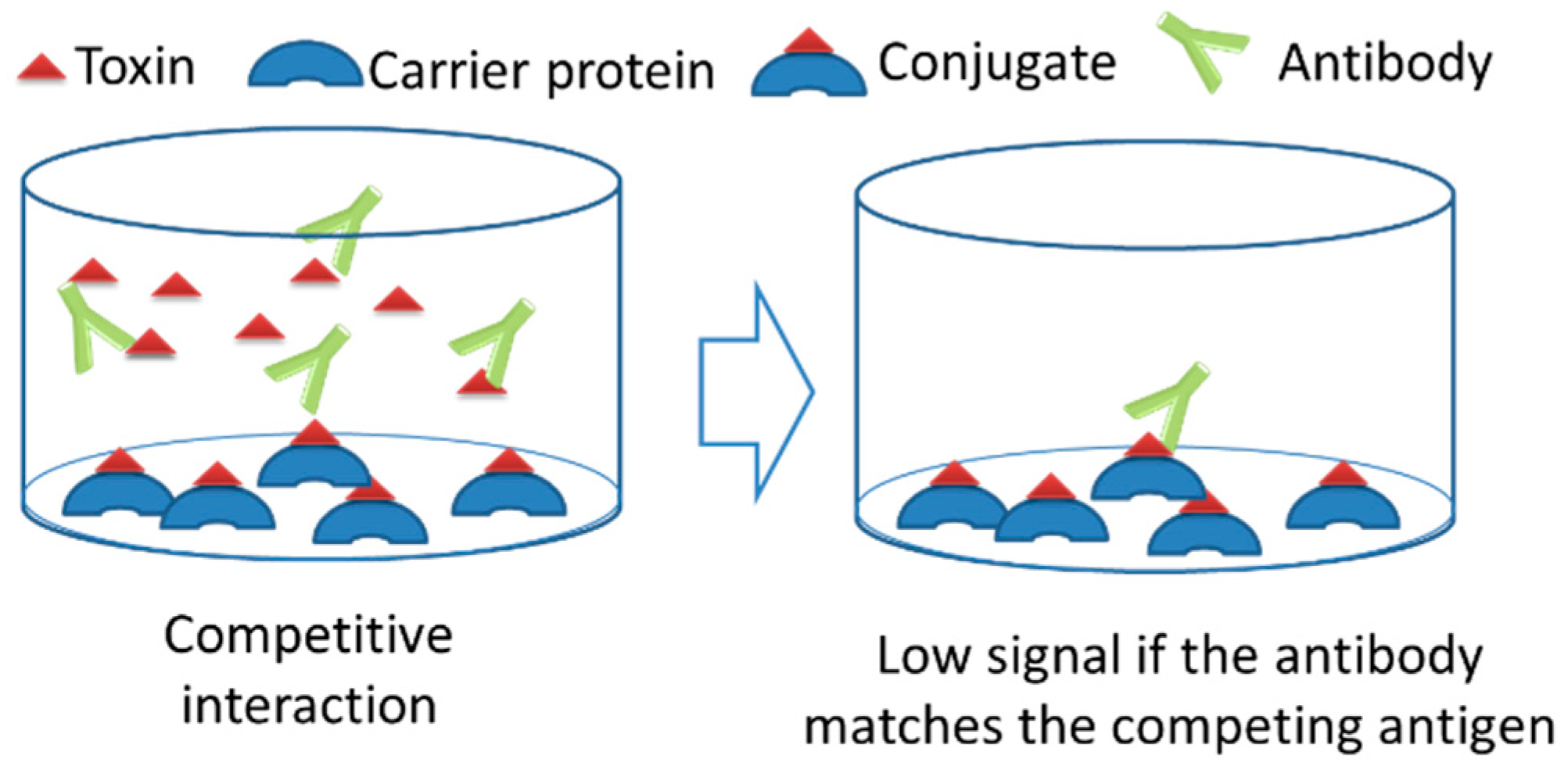

| Coating Antigen | Detected Antibodies | Competing Antigen | Blocked Antibodies | Signal Change |
|---|---|---|---|---|
 |  |  or or  | ||
| OVA-AFB1 or TF-AFB1 (conjugates used in immunizations) | AFB1 and carrier protein | AFB1 | Anti-AFB1 | Partial reduction |
| Carrier protein | Anti-carrier | Partial reduction | ||
| OVA or TF (Unconjugated carrier used in immunizations) | Carrier protein | AFB1 | None | No change |
| Carrier protein | Anti-carrier | No signal | ||
| BSA-AFB1 conjugate | AFB1 | AFB1 | Anti-AFB1 | No signal |
| Carrier protein | None | No change | ||
| BSA | Negative control | AFB1 | NA * | No signal |
| Carrier protein | NA * | No signal |
| Fusion | Immunogen | Myeloma Cells | Lymphocytes | Hybrid Cells | Antibody Producing Hybridomas |
|---|---|---|---|---|---|
| 1 | TF-AFB1 | 0.45 × 108 | 4 × 108 | 748 | 19 |
| 2 | TF-AFB1 | 2 × 108 | 5.92 × 108 | 878 | 31 |
| 3 | OVA-AFB1 | 2.4 × 108 | 19.6 × 108 | 1541 | 13 |
| 4 | OVA-AFB1 | 2.45 × 108 | 8.28 × 108 | 685 | 71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertekin, Ö.; Akçael, E.; Kocaağa, H.; Öztürk, S. Biological Activity of the Carrier as a Factor in Immunogen Design for Haptens. Molecules 2018, 23, 2977. https://doi.org/10.3390/molecules23112977
Ertekin Ö, Akçael E, Kocaağa H, Öztürk S. Biological Activity of the Carrier as a Factor in Immunogen Design for Haptens. Molecules. 2018; 23(11):2977. https://doi.org/10.3390/molecules23112977
Chicago/Turabian StyleErtekin, Özlem, Esin Akçael, Harun Kocaağa, and Selma Öztürk. 2018. "Biological Activity of the Carrier as a Factor in Immunogen Design for Haptens" Molecules 23, no. 11: 2977. https://doi.org/10.3390/molecules23112977





