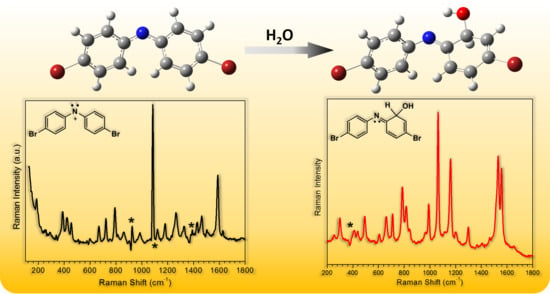
Time-Resolved Spectroscopic Study of N,N-Di(4-bromo)nitrenium Ions in Selected Solutions
Abstract
:1. Introduction
2. Results and Discussion
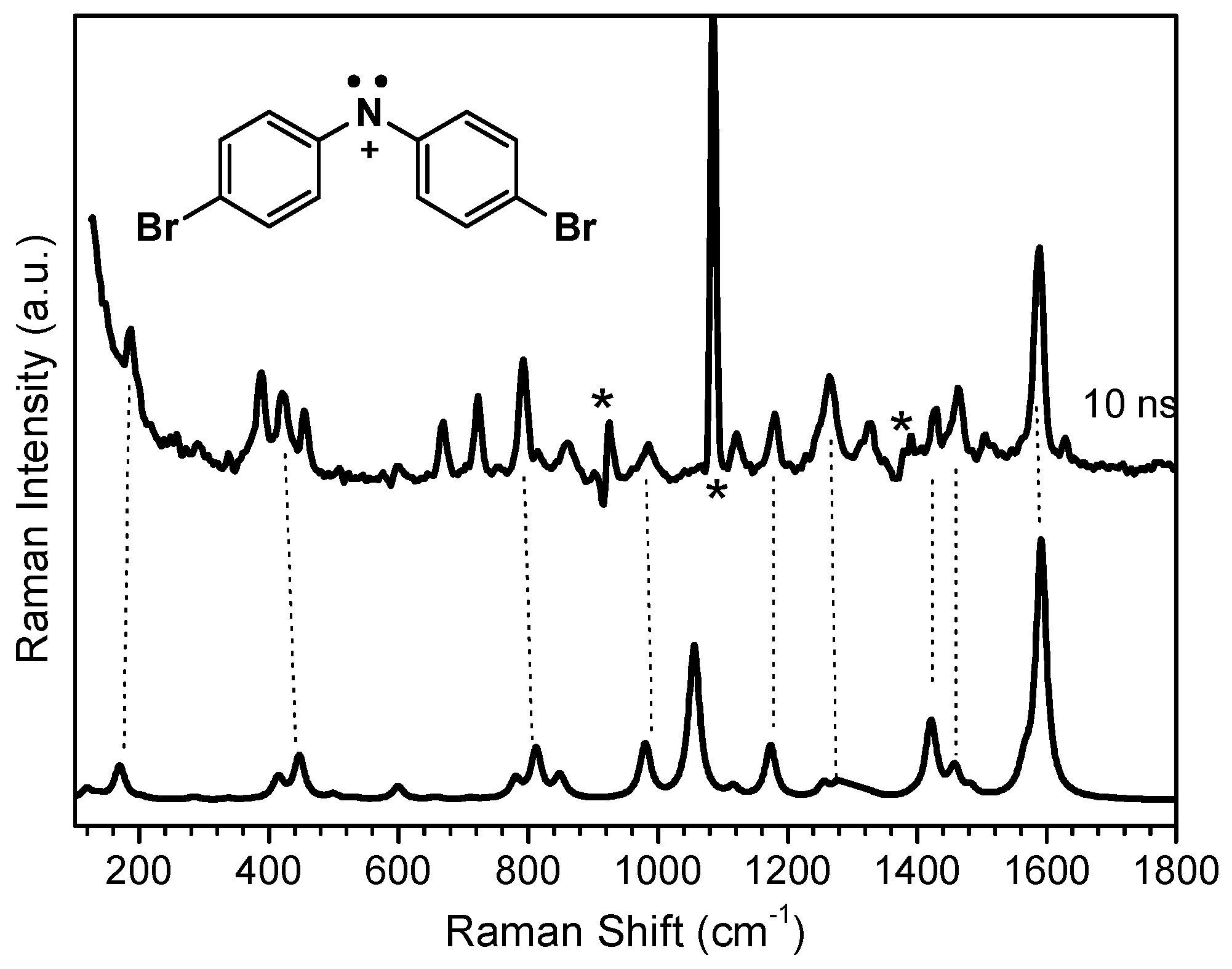
2.1. In MeCN
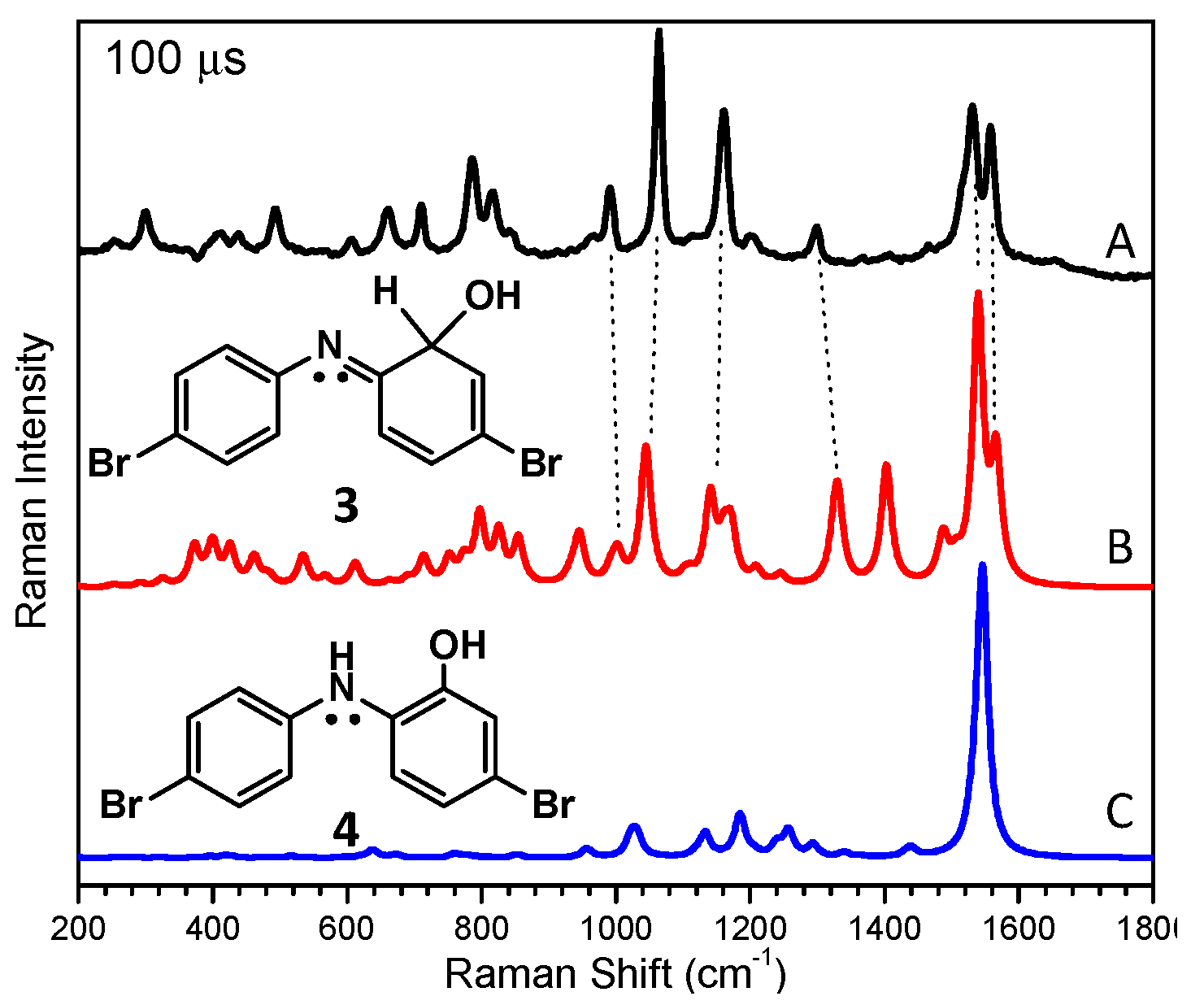
2.2. In Aqueous Solution
3. Materials and Methods
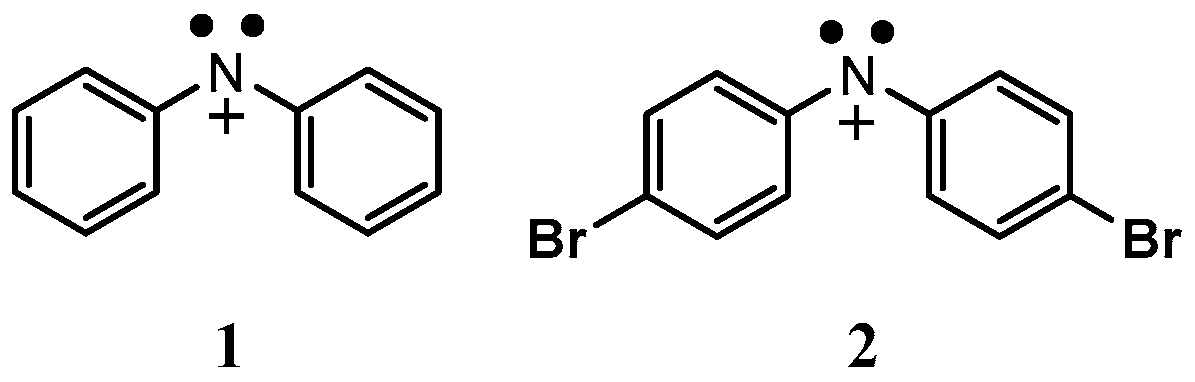
3.1. Materials
3.2. Experimental and Computational Methods
3.2.1. fs-TA and ns-TA Experiments
3.2.2. ns-TR3 Experiments
3.2.3. Density Functional Theory Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, E.C.; Lotlikar, P.D.; Miller, J.A.; Butler, B.W.; Irving, C.C.; Hill, J.T. Reactions in vitro of some tissue nucleophiles with the glucuronide of the carcinogen N-hydroxy-2-acetylaminofluorene. Mol. Pharmacol. 1968, 4, 147–154. [Google Scholar]
- Miller, E.C.; Miller, J.A. Mechanisms of chemical carcinogenesis: Nature of proximate carcinogens and interactions with macromolecules. Pharmacol. Rev. 1966, 18, 805–838. [Google Scholar]
- Poirier, L.A.; Miller, J.A.; Miller, E.C.; Sato, K. N-Benzoyloxy-N-methyl-4-aminoazobenzene: Its Carcinogenic Activity in the Rat and Its Reactions with Proteins and Nucleic Acids and Their Constituents in Vitro. Cancer Res. 1967, 27, 1600–1613. [Google Scholar]
- Novak, M.; Kennedy, S.A. Inhibitory effect of DNA structure on the efficiency of reaction of guanosine moieties with a nitrenium ion. J. Phys. Org. Chem. 1998, 11, 71–76. [Google Scholar] [CrossRef]
- Novak, M.; Kennedy, S.A. Selective Trapping of N-Acetyl-N-(4-biphenylyl)nitrenium and N-Acetyl-N-(2-fluorenyl)nitrenium Ions by 2′-Deoxyguanosine in Aqueous Solution. J. Am. Chem. Soc. 1995, 117, 574–575. [Google Scholar] [CrossRef]
- McClelland, R.A.; Ahmad, A.; Dicks, A.P.; Licence, V.E. Spectroscopic Characterization of the Initial C8 Intermediate in the Reaction of the 2-Fluorenylnitrenium Ion with 2′-Deoxyguanosine. J. Am. Chem. Soc. 1999, 121, 3303–3310. [Google Scholar] [CrossRef]
- Humphreys, W.G.; Kadlubar, F.F.; Guengerich, F.P. Mechanism of C8 alkylation of guanine residues by activated arylamines: Evidence for initial adduct formation at the N7 position. Proc. Natl. Acad. Sci. USA 1992, 89, 8278–8282. [Google Scholar] [CrossRef]
- McClelland, R.A.; Gadosy, T.A.; Ren, D. 1997 Alfred Bader Award Lecture Reactivities of arylnitrenium ions with guanine derivatives and other nucleophiles. Can. J. Chem. 1998, 76, 1327–1337. [Google Scholar] [CrossRef]
- Chan, P.Y.; Kwok, W.M.; Lam, S.K.; Chiu, P.; Phillips, D.L. Time-resolved resonance Raman observation of the 2-fluorenylnitrenium ion reaction with guanosine to form a C8 intermediate. J. Am. Chem. Soc. 2005, 127, 8246–8247. [Google Scholar] [CrossRef]
- Thomas, S.I.; Falvey, D.E. N,N-di(4-halophenyl)nitrenium ions: Nucleophilic trapping, aromatic substitution, and hydrogen atom transfer. J. Org. Chem. 2007, 72, 4626–4634. [Google Scholar] [CrossRef]
- McIlroy, S.; Moran, R.J.; Falvey, D.E. Photogenerated nitrenium ions: A search for triplet-state reactivity in the chemistry of the diphenylnitrenium ion. J. Phys. Chem. A 2000, 104, 11154–11158. [Google Scholar] [CrossRef]
- Moran, R.J.; Falvey, D.E. Photogenerated diarylnitrenium ions: Laser flash photolysis and product studies on diphenylnitrenium ion generated from photolysis of 1-(N,N-diphenylamino)pyridinium ions. J. Am. Chem. Soc. 1996, 118, 8965–8966. [Google Scholar] [CrossRef]
- Kung, A.C.; McIlroy, S.P.; Falvey, D.E. Diphenylnitrenium ion: Cyclization, electron transfer, and polymerization reactions. J. Org. Chem. 2005, 70, 5283–5290. [Google Scholar] [CrossRef]
- Srivastava, S.; Ruane, P.H.; Toscano, J.P.; Sullivan, M.B.; Cramer, C.J.; Chiapperino, D.; Reed, E.C.; Falvey, D.E. Structures of reactive nitrenium ions: Time-resolved infrared laser flash photolysis and computational studies of substituted N-methyl-N-arylnitrenium ions. J. Am. Chem. Soc. 2000, 122, 8271–8278. [Google Scholar] [CrossRef]
- Macernis, M.; Kietis, B.P.; Sulskus, J.; Lin, S.H.; Hayashi, M.; Valkunas, L. Triggering the proton transfer by H-bond network. Chem. Phys. Lett. 2008, 466, 223–226. [Google Scholar] [CrossRef]
- Kwok, W.M.; Chan, P.Y.; Phillips, D.L. Direct observation of the 2-fluorenylnitrene and 4-methoxyphenylnitrene reactions with water using picosecond Kerr-gated time-resolved resonance Raman spectroscopy. J. Phys. Chem. B 2004, 108, 19068–19075. [Google Scholar] [CrossRef]
- Srivastava, S.; Toscano, J.P.; Moran, R.J.; Falvey, D.E. Experimental confirmation of the iminocyclohexadienyl cation-like structure of arylnitrenium ions: Time-resolved IR studies of diphenylnitrenium ion. J. Am. Chem. Soc. 1997, 119, 11552–11553. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2006. [Google Scholar]
- Du, L.; Li, M.-D.; Zhang, Y.; Xue, J.; Zhang, X.; Zhu, R.; Cheng, S.C.; Li, X.; Phillips, D.L. Photoconversion of β-Lapachone to α-Lapachone via a Protonation-Assisted Singlet Excited State Pathway in Aqueous Solution: A Time-Resolved Spectroscopic Study. J. Org. Chem. 2015, 80, 7340–7350. [Google Scholar] [CrossRef]
- Du, L.L.; Zhu, R.X.; Xue, J.D.; Du, Y.; Phillips, D.L. Time-resolved spectroscopic and density functional theory investigation of the photochemistry of suprofen. J. Raman. Spectrosc. 2015, 46, 117–125. [Google Scholar] [CrossRef]
- Du, L.L.; Zhang, X.T.; Xue, J.D.; Tang, W.J.; Li, M.D.; Lan, X.; Zhu, J.R.; Zhu, R.X.; Weng, Y.X.; Li, Y.L.; et al. Influence of Water in the Photogeneration and Properties of a Bifunctional Quinone Methide. J. Phys. Chem. B 2016, 120, 11132–11141. [Google Scholar] [CrossRef]
- Du, L.; Qiu, Y.; Lan, X.; Zhu, R.; Phillips, D.L.; Li, M.-D.; Dutton, A.S.; Winter, A.H. Direct Detection of the Open-Shell Singlet Phenyloxenium Ion: An Atom-Centered Diradical Reacts as an Electrophile. J. Am. Chem. Soc. 2017, 139, 15054–15059. [Google Scholar] [CrossRef]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree-Fock, Moller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Merrick, J.P.; Moran, D.; Radom, L. An evaluation of harmonic vibrational frequency scale factors. J. Phys. Chem. A 2007, 111, 11683–11700. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Lan, X.; Yan, Z.; Zhu, R.; Phillips, D.L. Time-Resolved Spectroscopic Study of N,N-Di(4-bromo)nitrenium Ions in Selected Solutions. Molecules 2018, 23, 3182. https://doi.org/10.3390/molecules23123182
Du L, Lan X, Yan Z, Zhu R, Phillips DL. Time-Resolved Spectroscopic Study of N,N-Di(4-bromo)nitrenium Ions in Selected Solutions. Molecules. 2018; 23(12):3182. https://doi.org/10.3390/molecules23123182
Chicago/Turabian StyleDu, Lili, Xin Lan, Zhiping Yan, Ruixue Zhu, and David Lee Phillips. 2018. "Time-Resolved Spectroscopic Study of N,N-Di(4-bromo)nitrenium Ions in Selected Solutions" Molecules 23, no. 12: 3182. https://doi.org/10.3390/molecules23123182