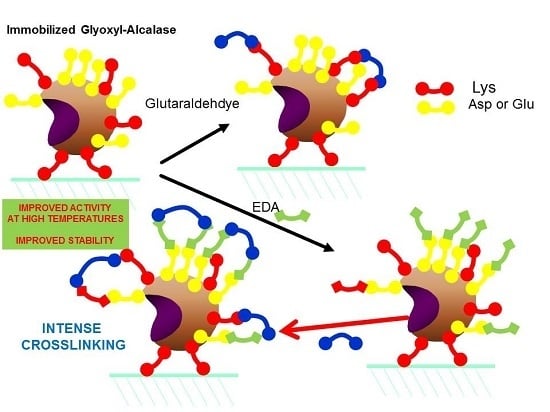
Further Stabilization of Alcalase Immobilized on Glyoxyl Supports: Amination Plus Modification with Glutaraldehyde
Abstract
:1. Introduction
2. Results
2.1. Immobilization of Alcalase in Glyoxyl Agarose (Glx-AL)
2.2. Effect of Temperature on the Hydrolysis of Casein by Different Alcalase Preparations
2.3. Modification with Ethylenediamine (EDA) of Alcalase Immobilized in Glyoxyl Agarose Beads (Glx-AL-EDA)
2.4. Modification with Glutaraldehyde of Alcalase Immobilized in Glyoxyl Agarose Beads (Glx-AL-GLU)
2.5. Modification of Glx-AL-EDA with Glutaraldehyde (Glx-AL-EDA-GLU)
2.6. Hydrolysis of Casein Using Glx-AL-EDA-GLU
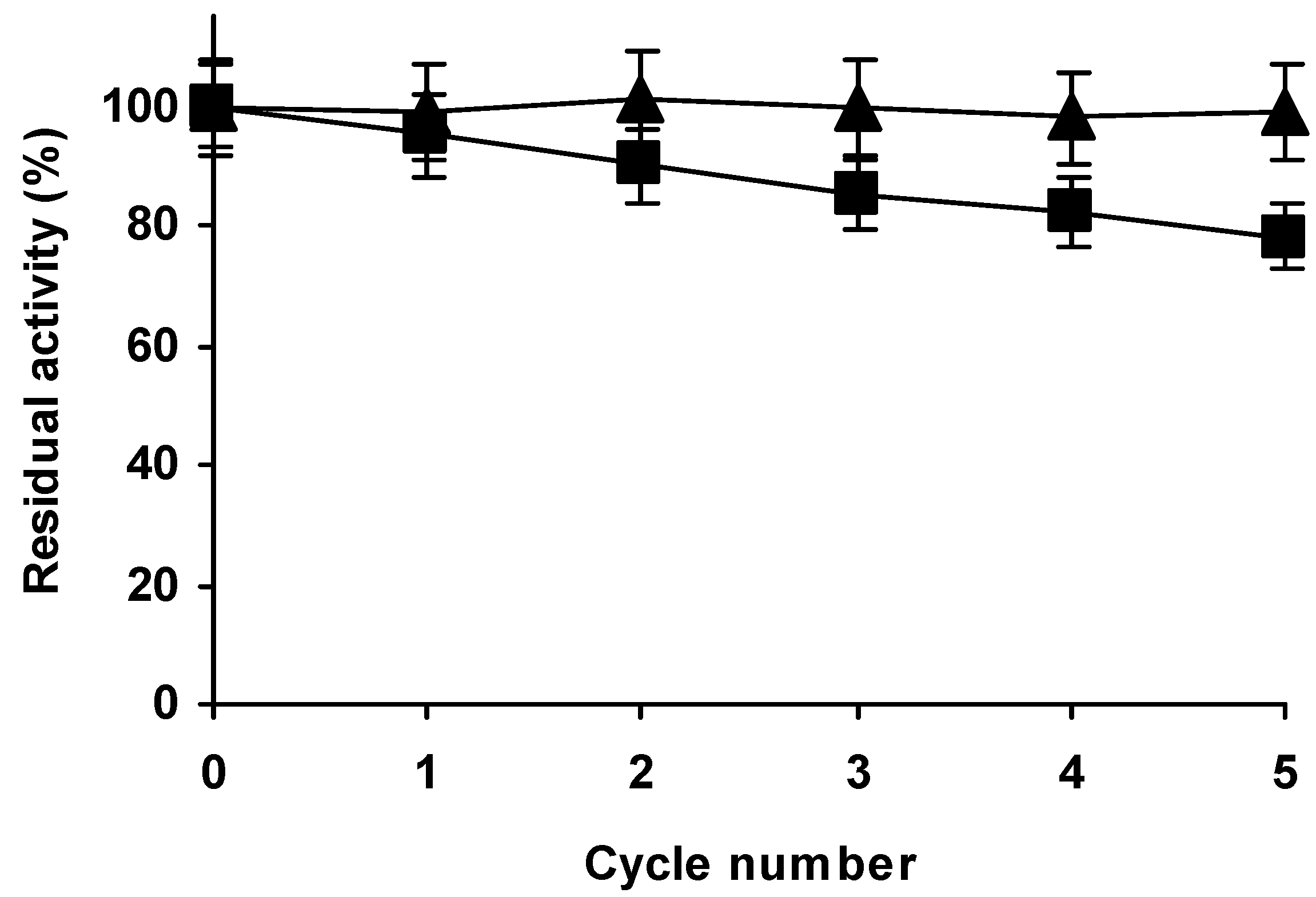
2.7. Reuse of Optimal Preparations at 45 °C and pH 9
3. Materials and Methods
3.1. Materials
3.2. Alcalase Activity Determination Using Synthetic Substrate
3.3. Alcalase Activity Determination Using Casein
3.4. Alcalase Immobilization on 4 BCL Glyoxyl-Agarose Beads
3.5. Immobilized Alcalase Amination
3.6. Alcalase Modification with Glutaraldehyde
3.7. Alcalase Inactivation
3.8. Immobilized Alcalase Reuses in Hydrolysis of Casein.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. [Google Scholar] [PubMed]
- Gupta, R.; Beg, Q.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002, 59, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Takagi, H. Microbial alkaline proteases: From a bioindustrial viewpoint. Biotechnol. Adv. 1999, 17, 561–594. [Google Scholar] [CrossRef]
- Barzkar, N.; Homaei, A.; Hemmati, R.; Patel, S. Thermostable marine microbial proteases for industrial applications: Scopes and risks. Extremophiles 2018, 22, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Contesini, F.J.; Melo, R.R.D.; Sato, H.H. An overview of Bacillus proteases: From production to application. Crit. Rev. Biotechnol. 2018, 38, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Mamo, J.; Assefa, F. The role of microbial aspartic protease enzyme in food and beverage industries. J. Food Qual. 2018, 2018, 7957269. [Google Scholar] [CrossRef]
- Dos Santos Aguilar, J.G.; Sato, H.H. Microbial proteases: Production and application in obtaining protein hydrolysates. Food Res. Int. 2018, 103, 253–262. [Google Scholar] [CrossRef]
- Tavano, O.L. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Catal. B 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of ace inhibitory peptides by digestion of chickpea legumin with Alcalase. Food Chem. 2003, 81, 363–369. [Google Scholar] [CrossRef]
- Adamson, N.J.; Reynolds, E.C. Characterization of casein phosphopeptides prepared using alcalase: Determination of enzyme specificity. Enzyme Microb. Technol. 1996, 19, 202–207. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, S.; Xu, J.; Zeng, M.; Song, H.; Zhao, Y. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control 2008, 19, 231–235. [Google Scholar] [CrossRef]
- Chen, S.-T.; Chen, S.-Y.; Wang, K.-T. Kinetically controlled peptide bond formation in anhydrous alcohol catalyzed by the industrial protease Alcalase. J. Org. Chem. 1992, 57, 6960–6965. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Jeon, Y.-J.; Kim, Y.-T.; Je, J.-Y. Angiotensin i converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Yust, M.d.M.; Pedroche, J.; Millán-Linares, M.d.C.; Alcaide-Hidalgo, J.M.; Millán, F. Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chem. 2010, 122, 1212–1217. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, T.-H.; Sun, M.-J. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J. Funct. Foods 2014, 7, 191–200. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Wu, Q.; Du, J.; Jia, J.; Kuang, C. Production of ACE inhibitory peptides from sweet sorghum grain protein using alcalase: Hydrolysis kinetic, purification and molecular docking study. Food Chem. 2016, 199, 140–149. [Google Scholar] [CrossRef]
- Dey, S.S.; Dora, K.C. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus). J. Food Sci. Technol. 2014, 51, 449–457. [Google Scholar] [CrossRef]
- Jia, J.; Wu, Q.; Yan, H.; Gui, Z. Purification and molecular docking study of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide from alcalase hydrolysate of ultrasonic-pretreated silkworm pupa (Bombyx mori) protein. Process Biochem. 2015, 50, 876–883. [Google Scholar] [CrossRef]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; De Oliveira, D. Nanomaterials for biocatalyst immobilization-state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N.; Cui, J. “Smart” chemistry and its application in peroxidase immobilization using different support materials. Int. J. Biol. Macromol. 2018, 119, 278–290. [Google Scholar] [CrossRef]
- Balcão, V.M.; Vila, M.M.D.C. Structural and functional stabilization of protein entities: State-of-the-art. Adv. Drug Deliv. Rev. 2015, 93, 25–41. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties- Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef]
- Bahamondes, C.; Illanes, A. Effect of internal diffusional restrictions on the selectivity of α-chymotrypsin in a series-parallel reaction of peptide synthesis. Process Biochem. 2018, 68, 117–120. [Google Scholar] [CrossRef]
- Bahamondes, C.; Álvaro, G.; Wilson, L.; Illanes, A. Effect of enzyme load and catalyst particle size on the diffusional restrictions in reactions of synthesis and hydrolysis catalyzed by α-chymotrypsin immobilized into glyoxal-agarose. Process Biochem. 2017, 53, 172–179. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Long, C.; Xia, J.; Tong, P.; Cheng, Y.; Wang, Y.; Chen, H. Enzymatic characterisation of the immobilised Alcalase to hydrolyse egg white protein for potential allergenicity reduction. J. Sci. Food Agric. 2017, 97, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Meng, T.; Ma, H.; Zhang, Y.; Li, Y.; Jin, J.; Ye, X. Mechanism study of dual-frequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrason. Sonochem. 2016, 32, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pessato, T.B.; de Carvalho, N.C.; Tavano, O.L.; Fernandes, L.G.R.; Zollner, R.D.L.; Netto, F.M. Whey protein isolate hydrolysates obtained with free and immobilized Alcalase: Characterization and detection of residual allergens. Food Res. Int. 2016, 83, 112–120. [Google Scholar] [CrossRef]
- Corîci, L.N.; Frissen, A.E.; Van Zoelen, D.-J.; Eggen, I.F.; Peter, F.; Davidescu, C.M.; Boeriu, C.G. Sol-gel immobilization of Alcalase from Bacillus licheniformis for application in the synthesis of C-terminal peptide amides. J. Mol. Catal. B Enzym. 2011, 73, 90–97. [Google Scholar] [CrossRef]
- Tardioli, P.W.; Sousa, R., Jr.; Giordano, R.C.; Giordano, R.L.C. Kinetic model of the hydrolysis of polypeptides catalyzed by Alcalase® immobilized on 10% glyoxyl-agarose. Enzyme Microb. Technol. 2005, 36, 555–564. [Google Scholar] [CrossRef]
- Tardioli, P.W.; Pedroche, J.; Giordano, R.L.C.; Fernández-Lafuente, R.; Guisán, J.M. Hydrolysis of proteins by immobilized-stabilized Alcalase-glyoxyl agarose. Biotechnol. Prog. 2003, 19, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Ó’Fágáin, C. Enzyme stabilization—Recent experimental progress. Enzyme Microb. Technol. 2003, 33, 137–149. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Wong, L.-J.C. Chemical crosslinking and the stabilization of proteins and enzymes. Enzyme Microb. Technol. 1992, 14, 866–874. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Chemical modification in the design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Chem. Rec. 2016, 16, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.M.; Knowles, J.R. Glutaraldehyde as a protein cross-linking reagent. J. Mol. Biol. 1968, 37, 231–233. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Wine, Y.; Cohen-Hadar, N.; Freeman, A.; Frolow, F. Elucidation of the mechanism and end products of glutaraldehyde crosslinking reaction by X-ray structure analysis. Biotechnol. Bioeng. 2007, 98, 711–718. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Guisan, J.M. Strategies for enzyme stabilization by intramolecular crosslinking with bifunctional reagents. Enzyme Microb. Technol. 1995, 17, 517–523. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R. Chemical amination of lipases improves their immobilization on octyl-glyoxyl agarose beads. Catal. Today 2016, 259, 107–118. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Godoy, C.A.; Volpato, G.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization-stabilization of the lipase from Thermomyces lanuginosus: Critical role of chemical amination. Process Biochem. 2009, 44, 963–968. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Godoy, C.A.; Mendes, A.A.; Lopez-Gallego, F.; Grazu, V.; de las Rivas, B.; Palomo, J.M.; Hermoso, J.; Fernandez-Lafuente, R.; Guisan, J.M. Solid-phase chemical amination of a lipase from Bacillus thermocatenulatus to improve its stabilization via covalent immobilization on highly activated glyoxyl-agarose. Biomacromolecules 2008, 9, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- De Morais Júnior, W.G.; Terrasan, C.R.F.; Fernández-Lorente, G.; Guisán, J.M.; Ribeiro, E.J.; de Resende, M.M.; Pessela, B.C. Solid-phase amination of Geotrichum candidum lipase: Ionic immobilization, stabilization and fish oil hydrolysis for the production of Omega-3 polyunsaturated fatty acids. Eur. Food Res. Technol. 2017, 243, 1375–1384. [Google Scholar] [CrossRef]
- Pereira, M.G.; Facchini, F.D.A.; Polizeli, A.M.; Vici, A.C.; Jorge, J.A.; Pessela, B.C.; Férnandez-Lorente, G.; Guisán, J.M.; De Moraes Polizeli, M.D.L.T. Stabilization of the lipase of Hypocrea pseudokoningii by multipoint covalent immobilization after chemical modification and application of the biocatalyst in oil hydrolysis. J. Mol. Catal. B: Enzym. 2015, 121, 82–89. [Google Scholar] [CrossRef]
- Martins de Oliveira, S.; Moreno-Perez, S.; Romero-Fernández, M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Guisan, J.M. Immobilization and stabilization of commercial β-1,4-endoxylanase Depol™ 333MDP by multipoint covalent attachment for xylan hydrolysis: Production of prebiotics (xylo-oligosaccharides). Biocatal. Biotransform. 2018, 36, 141–150. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Solid phase chemical modification of agarose glyoxyl-ficin: Improving activity and stability properties by amination and modification with glutaraldehyde. Process Biochem. 2018, 73, 109–116. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Dos Santos, J.C.S.; Barbosa, O.; Torres, R.; Pereira, E.B.; Corberan, V.C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning of Lecitase features via solid-phase chemical modification: Effect of the immobilization protocol. Process Biochem. 2014, 49, 604–616. [Google Scholar] [CrossRef]
- Barbosa, O.; Ruiz, M.; Ortiz, C.; Fernández, M.; Torres, R.; Fernandez-Lafuente, R. Modulation of the properties of immobilized CALB by chemical modification with 2,3,4-trinitrobenzenesulfonate or ethylendiamine. Advantages of using adsorbed lipases on hydrophobic supports. Process Biochem. 2012, 47, 867–876. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Effect of solid-phase chemical modification on the features of the lipase from Thermomyces lanuginosus. Process Biochem. 2012, 47, 460–466. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fernández-Lorente, G.; Guisán, J.M.; Fernández-Lafuente, R. Modulation of immobilized lipase enantioselectivity via chemical amination. Adv. Synth. Cat. 2007, 349, 1119–1127. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Ruiz, M.; Cruz, J.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Chemical amination of lipase B from Candida antarctica is an efficient solution for the preparation of crosslinked enzyme aggregates. Process Biochem. 2012, 47, 2373–2378. [Google Scholar] [CrossRef]
- Ruiz, M.; Galvis, M.; Barbosa, O.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Solid-phase modification with succinic polyethyleneglycol of aminated lipase B from Candida antarctica: Effect of the immobilization protocol on enzyme catalytic properties. J. Mol. Catal. B 2013, 87, 75–82. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Bernedo, M.; Cuenca, E.; Fuentes, M.; Fernandez-Lorente, G.; Palomo, J.M.; Grazu, V.; Pessela, B.C.C.; Giacomini, C.; et al. Some special features of glyoxyl supports to immobilize proteins. Enzyme Microb. Technol. 2005, 37, 456–462. [Google Scholar] [CrossRef]
- Abian, O.; Grazú, V.; Hermoso, J.; González, R.; García, J.L.; Fernández-Lafuente, R.; Guisán, J.M. Stabilization of Penicillin G Acylase from Escherichia coli: Site-Directed Mutagenesis of the Protein Surface to Increase Multipoint Covalent Attachment. Appl. Environ. Microbiol. 2004, 70, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Siar, E.-H.; Zaak, H.; Kornecki, J.F.; Zidoune, M.N.; Barbosa, O.; Fernandez-Lafuente, R. Stabilization of ficin extract by immobilization on glyoxyl agarose. Preliminary characterization of the biocatalyst performance in hydrolysis of proteins. Process Biochem. 2017, 58, 98–104. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Immobilization/stabilization of ficin extract on glutaraldehyde-activated agarose beads. Variables that control the final stability and activity in protein hydrolyses. Catalysts 2018, 8. [Google Scholar] [CrossRef]
- Pedroche, J.; del Mar Yust, M.; Mateo, C.; Fernández-Lafuente, R.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Guisán, J.M.; Millán, F. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: Correlation between enzyme-support linkages and thermal stability. Enzyme Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Woodley, J. Implications of enzyme stability for biocatalytic process development, design and economics. In Proceedings of the 11th International Conference on Protein stabilization, Istambul, Turkey, 9–11 May 2016. [Google Scholar]
- Kunitz, M. Crystalline soybean trypsin inhibitor. J. Gen. Physiol. 1946, 29, 149–154. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzyme Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and its derivatives as supports for enzyme immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L.; Koshland, D.E. Carbodiimide modification of proteins. Methods Enzymol. 1972, 25, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L.; Spoerl, P.; Koshland, D.E. Carboxyl group modification in chymotrypsin and chymotrypsinogen. J. Mol. Biol. 1969, 42, 133–137. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, F.; Arana-Peña, S.; Morellon-Sterling, R.; Barbosa, O.; Ait Braham, S.; Kamal, S.; Fernandez-Lafuente, R. Further Stabilization of Alcalase Immobilized on Glyoxyl Supports: Amination Plus Modification with Glutaraldehyde. Molecules 2018, 23, 3188. https://doi.org/10.3390/molecules23123188
Hussain F, Arana-Peña S, Morellon-Sterling R, Barbosa O, Ait Braham S, Kamal S, Fernandez-Lafuente R. Further Stabilization of Alcalase Immobilized on Glyoxyl Supports: Amination Plus Modification with Glutaraldehyde. Molecules. 2018; 23(12):3188. https://doi.org/10.3390/molecules23123188
Chicago/Turabian StyleHussain, Fouzia, Sara Arana-Peña, Roberto Morellon-Sterling, Oveimar Barbosa, Sabrina Ait Braham, Shagufta Kamal, and Roberto Fernandez-Lafuente. 2018. "Further Stabilization of Alcalase Immobilized on Glyoxyl Supports: Amination Plus Modification with Glutaraldehyde" Molecules 23, no. 12: 3188. https://doi.org/10.3390/molecules23123188