Design and Properties of Ligand-Conjugated Guanine Oligonucleotides for Recovery of Mutated G-Quadruplexes
Abstract
:1. Introduction
2. Results
2.1. Design of Ligand-Conjugated Guanine Tracts
2.2. Structures of Intermolecular G4s of Mutated VEGF with Ligand-Conjugated Guanine Tracts
2.3. Stability of Intermolecular G4s of Mutated VEGF with Ligand-Conjugated Guanine Tracts
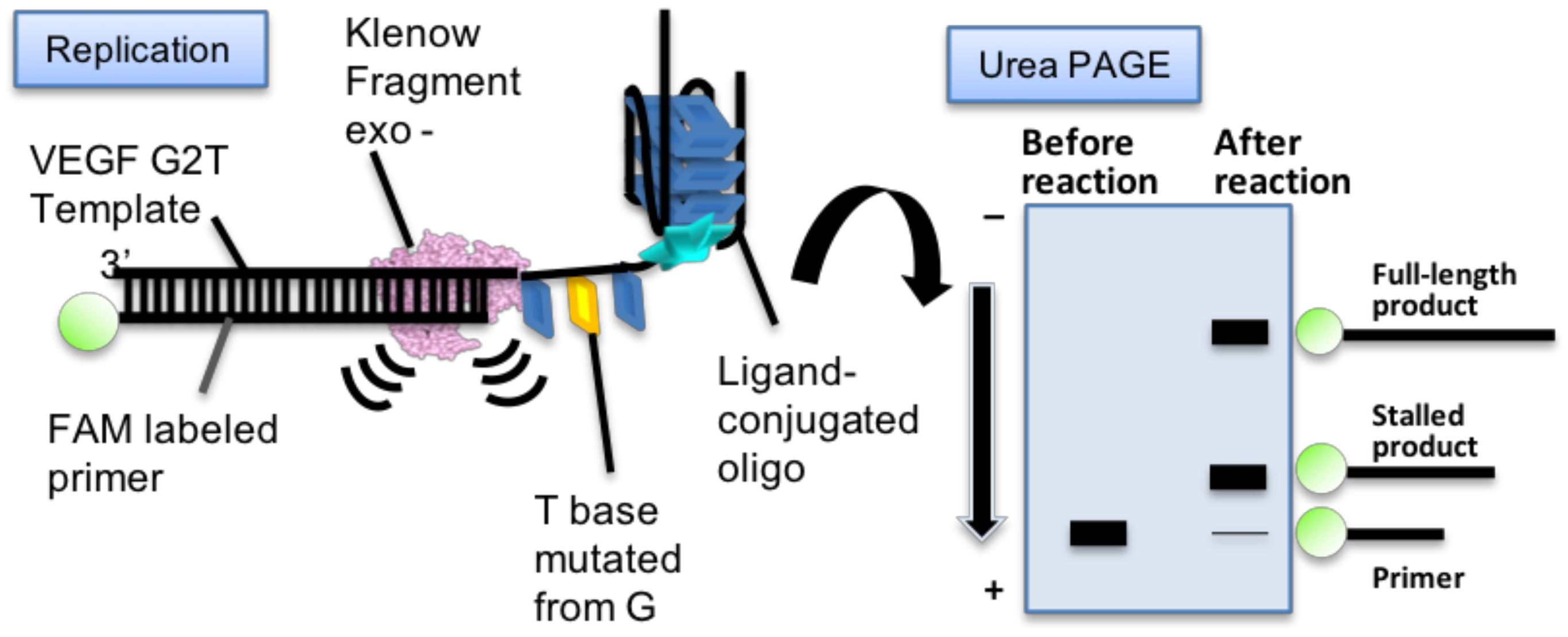
2.4. Replication Assay of Mutated VEGF G4 with Ligand-Conjugated Guanine Tracts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Oligonucleotides
4.3. CD Spectrum Acquisition
4.4. CD Melting Assay
4.5. Replication Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Rhodes, D.; Lipps, H.J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansel-Hertsch, R.; Di Antonio, M.; Balasubramanian, S. DNA G-quadruplexes in the human genome: Detection, functions and therapeutic potential. Nat. Rev. Mol. Cell Biol. 2017, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Miyoshi, D.; Sugimoto, N. Effects of molecular crowding on the structures, interactions, and functions of nucleic acids. Chem. Rev. 2014, 114, 2733–2758. [Google Scholar] [CrossRef] [PubMed]
- Sekibo, D.A.T.; Fox, K.R. The effects of DNA supercoiling on G-quadruplex formation. Nucleic Acids Res. 2017, 45, 12069–12079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateishi-Karimata, H.; Kawauchi, K.; Sugimoto, N. Destabilization of DNA G-Quadruplexes by Chemical Environment Changes during Tumor Progression Facilitates Transcription. J. Am. Chem. Soc. 2018, 140, 642–651. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Delaney, S.; Barton, J.K. Charge transport in DNA duplex/quadruplex conjugates. Biochemistry 2003, 42, 14159–14165. [Google Scholar] [CrossRef]
- Pastukh, V.; Roberts, J.T.; Clark, D.W.; Bardwell, G.C.; Patel, M.; Al-Mehdi, A.B.; Borchert, G.M.; Gillespie, M.N. An oxidative DNA “damage” and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1367–L1375. [Google Scholar] [CrossRef]
- Fleming, A.M.; Ding, Y.; Burrows, C.J. Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc. Natl. Acad. Sci. USA 2017, 114, 2604–2609. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Kim, K.T.; Podbevsek, P.; Plavec, J.; Kim, B.H.; Sugimoto, N. Recovery of the Formation and Function of Oxidized G-Quadruplexes by a Pyrene-Modified Guanine Tract. J. Am. Chem. Soc. 2018, 140, 5774–5783. [Google Scholar] [CrossRef]
- Van Loon, B.; Markkanen, E.; Hubscher, U. Oxygen as a friend and enemy: How to combat the mutational potential of 8-oxo-guanine. DNA Repair 2010, 9, 604–616. [Google Scholar] [CrossRef]
- Park, Y.; Kim, K.T.; Kim, B.H. G-Quadruplex formation using fluorescent oligonucleotides as a detection method for discriminating AGG trinucleotide repeats. Chem. Commun. 2016, 52, 12757–12760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doluca, O.; Withers, J.M.; Loo, T.S.; Edwards, P.J.; Gonzalez, C.; Filichev, V.V. Interdependence of pyrene interactions and tetramolecular G4-DNA assembly. Org. Biomol. Chem. 2015, 13, 3742–3748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaluzhny, D.; Ilyinsky, N.; Shchekotikhin, A.; Sinkevich, Y.; Tsvetkov, P.O.; Tsvetkov, V.; Veselovsky, A.; Livshits, M.; Borisova, O.; Shtil, A.; et al. Disordering of human telomeric G-quadruplex with novel antiproliferative anthrathiophenedione. PLoS ONE 2011, 6, e27151. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cliff, C.L.; Hurley, L.H. Accelerated assembly of G-quadruplex structures by a small molecule. Biochemistry 1999, 38, 6981–6986. [Google Scholar] [CrossRef] [PubMed]
- Dioubankova, N.N.; Malakhov, A.D.; Stetsenko, D.A.; Gait, M.J.; Korshun, V.A. Phosphoramidites and solid supports based on N-substituted 2, 4-dihydroxybutyramides: Universal reagents for synthesis of modified oligonucleotides. Tetrahedron 2006, 62, 6762–6773. [Google Scholar] [CrossRef]
- Stetsenko, D.A.; Gait, M.J. A convenient solid-phase method for synthesis of 3′-conjugates of oligonucleotides. Bioconjug. Chem. 2001, 12, 576–586. [Google Scholar] [CrossRef]
- Malakhov, A.D.; Skorobogatyi, M.V.; Prokhorenko, I.A.; Gontarev, S.V.; Kozhich, D.T.; Stetsenko, D.A.; Stepanova, I.A.; Shenkarev, Z.O.; Berlin, Y.A.; Korshun, V.A. 1-(Phenylethynyl) pyrene and 9, 10-Bis (phenylethynyl) anthracene, Useful Fluorescent Dyes for DNA Labeling: Excimer Formation and Energy Transfer. Eur. J. Org. Chem. 2004, 2004, 1298–1307. [Google Scholar] [CrossRef]
- Dai, J.; Carver, M.; Hurley, L.H.; Yang, D. Solution structure of a 2:1 quindoline-c-MYC G-quadruplex: Insights into G-quadruplex-interactive small molecule drug design. J. Am. Chem. Soc. 2011, 133, 17673–17680. [Google Scholar] [CrossRef]
- Kochoyan, M.; Lancelot, G.; Leroy, J.L. Study of structure, base-pair opening kinetics and proton exchange mechanism of the d-(AATTGCAATT) self-complementary oligodeoxynucleotide in solution. Nucleic Acids Res. 1988, 16, 7685–7702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateishi-Karimata, H.; Ohyama, T.; Muraoka, T.; Podbevsek, P.; Wawro, A.M.; Tanaka, S.; Nakano, S.I.; Kinbara, K.; Plavec, J.; Sugimoto, N. Newly characterized interaction stabilizes DNA structure: Oligoethylene glycols stabilize G-quadruplexes CH-pi interactions. Nucleic Acids Res. 2017, 45, 7021–7030. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, D.; Guilbaud, G.; Murat, P.; Papadopoulou, C.; Sarkies, P.; Prioleau, M.N.; Balasubramanian, S.; Sale, J.E. Determinants of G quadruplex-induced epigenetic instability in REV1-deficient cells. EMBO J. 2014, 33, 2507–2520. [Google Scholar] [CrossRef] [PubMed]
- Damha, M.J.; Giannaris, P.A.; Zabarylo, S.V. An improved procedure for derivatization of controlled-pore glass beads for solid-phase oligonucleotide synthesis. Nucleic Acids Res. 1990, 18, 3813–3821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dioubankova, N.N.; Malakhov, A.D.; Stetsenko, D.A.; Korshun, V.A.; Gait, M.J. (R)-2,4-Dihydroxybutyramide seco-pseudonucleosides: New versatile homochiral synthons for synthesis of modified oligonucleotides. Org. Lett. 2002, 4, 4607–4610. [Google Scholar] [CrossRef] [PubMed]
- Prokhorenko, I.A.; Korshun, V.A.; Petrov, A.A.; Gontarev, S.V.; Berlin, Y.A. Incorporation of a pyrene nucleoside analogue into synthetic oligodeoxynucleotides using a nucleoside-like synthon. Bioorg. Med. Chem. Lett. 1995, 5, 2081–2084. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| Name | Sequence (5′→ 3′) |
|---|---|
| VEGF G2T | CAGTGCGGGCCTTGGGCGGGAT |
| VEGF G2T template | CAGTGCGGGCCTTGGGCGGGATCGGACCTATAGTGAGTCGTATTCCC |
| FAM labeled primer | [FITC]-GGGAATACGACTCACTATAGG |
| Ligand-Conjugated Guanine Tract | Tm (°C) | −∆G°37 (kcal mol−1) |
|---|---|---|
| PySG3 | 50.7 | 2.6 ± 0.4 |
| G3PyS | 51.6 | 2.7 ± 0.4 |
| PyLG3 | 53.5 | 3.3 ± 0.5 |
| G3PyL | 55.5 | 3.5 ± 0.5 |
| PEPyG3 | 58.4 | 3.6 ± 0.6 |
| G3PEPy | 65.3 | 3.7 ± 2.1 |
| BPEAG3 | n.d. | n.d. |
| G3BPEA | n.d. | n.d. |
| PerG3 | 59.7 | 3.3 ± 0.8 |
| G3Per | 62.1 | 3.7 ± 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, S.; Chelobanov, B.; Kim, K.T.; Kim, B.H.; Stetsenko, D.; Sugimoto, N. Design and Properties of Ligand-Conjugated Guanine Oligonucleotides for Recovery of Mutated G-Quadruplexes. Molecules 2018, 23, 3228. https://doi.org/10.3390/molecules23123228
Takahashi S, Chelobanov B, Kim KT, Kim BH, Stetsenko D, Sugimoto N. Design and Properties of Ligand-Conjugated Guanine Oligonucleotides for Recovery of Mutated G-Quadruplexes. Molecules. 2018; 23(12):3228. https://doi.org/10.3390/molecules23123228
Chicago/Turabian StyleTakahashi, Shuntaro, Boris Chelobanov, Ki Tae Kim, Byeang Hyean Kim, Dmitry Stetsenko, and Naoki Sugimoto. 2018. "Design and Properties of Ligand-Conjugated Guanine Oligonucleotides for Recovery of Mutated G-Quadruplexes" Molecules 23, no. 12: 3228. https://doi.org/10.3390/molecules23123228






