Cardioprotective Effects of Puerarin-V on Isoproterenol-Induced Myocardial Infarction Mice Is Associated with Regulation of PPAR-Υ/NF-κB Pathway
Abstract
:1. Introduction
2. Results
2.1. Puerarin-V Improved Cardiac Function and Suppressed Mortality in the MI Mice
2.2. Puerarin-V Treatment Suppressed the Myocardial Inflammation and Necrosis in the MI Mice
2.3. Puerarin-V Alleviated Inflammation Injury in the MI Mice
2.4. Puerarin-V Inhibited Myocardial Apoptosis in the MI Mice
2.5. Puerarin-V Attenuated ISO-Induced Inflammation in the MI Mice Associated with Upregulation of PPAR-γ Expression and Inhibition of NF-κB Phosphorylation
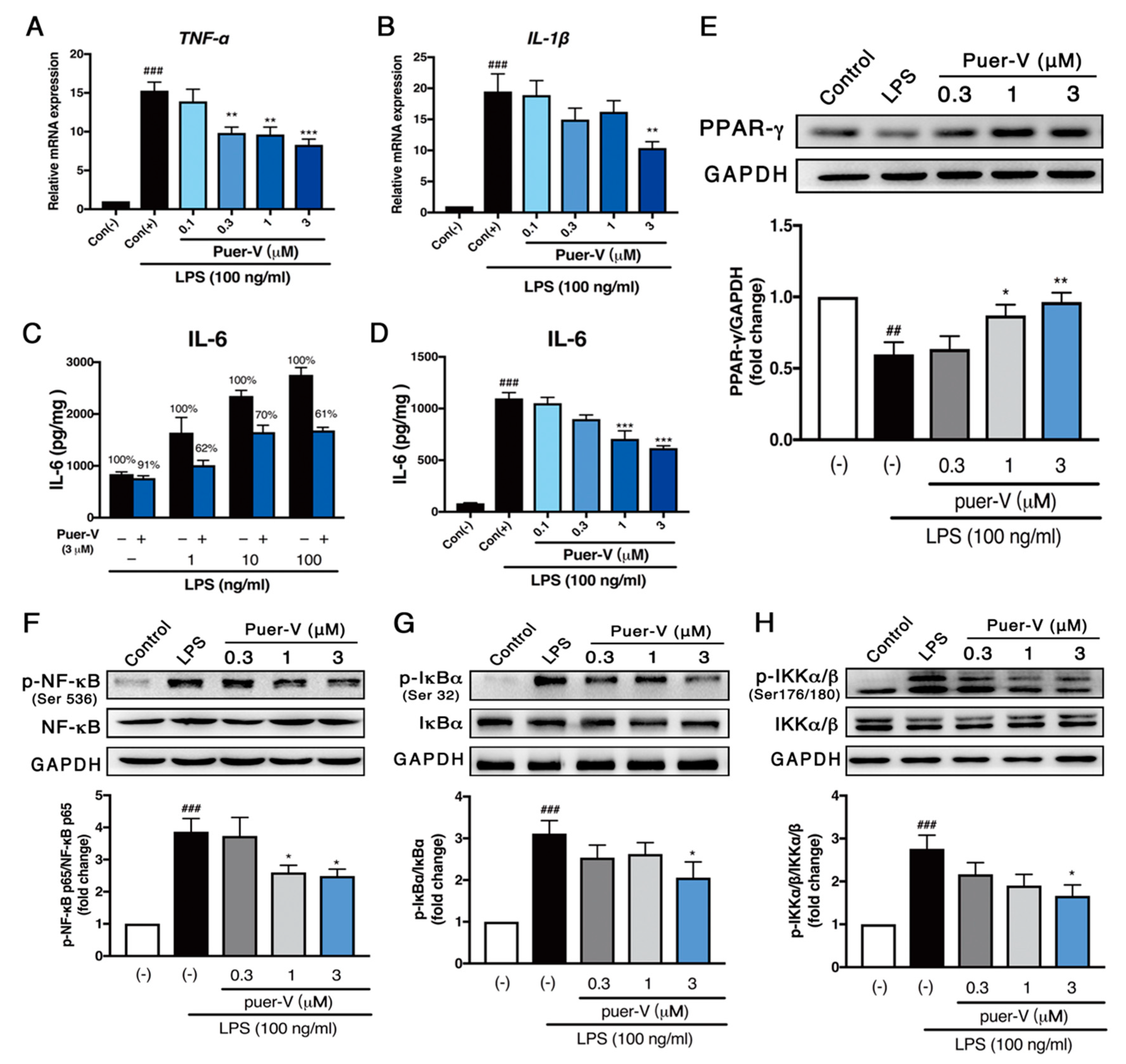
2.6. Puerarin-V Protected Coronary Artery Endothelial Cells Function against LPS-Induced Inflammation Associated with Upregulation of PPAR-γ Expression and Inhibition of NF-κB Phosphorylation
2.7. Puerarin-V Protected against LPS-Induced Cell Apoptosis in HCAECs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and Experimental Design
4.3. Electrocardiogram Recording
4.4. TTC Staining
4.5. Determination of AST, LDH and cTn-T Release into Serum
4.6. Measurement of Inflammatory Cytokines by ELISA
4.7. H&E Staining and Immunohistochemical Analysis of Macrophage Marker CD68
4.8. Western Blot Analysis
4.9. Total RNA Extraction for Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.10. Determination of Myocardial Apoptosis
4.11. HCAEC Culture
4.12. Cell Viability Assay
4.13. Stimulation of Cells with LPS and IL-6 Measurements
4.14. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Allawadhi, P.; Khurana, A.; Sayed, N.; Kumari, P.; Godugu, C. Isoproterenol-induced cardiac ischemia and fibrosis: Plant-based approaches for intervention. Phytother. Res. 2018, 32, 1908–1932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Zhou, C.; Jia, H.M.; Chang, X.; Zou, Z.M. Standardized Chinese Formula Xin-Ke-Shu inhibits the myocardium Ca(2+) overloading and metabolic alternations in isoproterenol-induced myocardial infarction rats. Sci. Rep. 2016, 6, 30208. [Google Scholar] [CrossRef] [PubMed]
- Raish, M. Momordica charantia polysaccharides ameliorate oxidative stress, hyperlipidemia, inflammation, and apoptosis during myocardial infarction by inhibiting the NF-κB signaling pathway. Int. J. Biol. Macromol. 2017, 97, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, H.; Zhao, J.; Wang, Y. Osmotin Protects H9c2 Cells from Simulated Ischemia-Reperfusion Injury through AdipoR1/PI3K/AKT Signaling Pathway. Front. Physiol. 2017, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Yang, Z.; Yuan, Y.; Wu, Q.Q.; Xu, M.; Jin, Y.G.; Tang, Q.Z. Sesamin prevents apoptosis and inflammation after experimental myocardial infarction by JNK and NF-κB pathways. Food Funct. 2017, 8, 2875–2885. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.P.; Wang, Y.; Zhang, Q.H.; Guo, J.; Li, L.; Cao, Y.G.; Li, S.Z.; Li, X.L.; Shi, M.M.; Xu, W.; et al. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) through NF-κB/Brg1 and TGF-beta1 pathways attenuates cardiac remodeling in pressure-overloaded rat hearts. Cell Physiol. Biochem. 2015, 35, 899–912. [Google Scholar] [CrossRef]
- Lv, F.H.; Yin, H.L.; He, Y.Q.; Wu, H.M.; Kong, J.; Chai, X.Y.; Zhang, S.R. Effects of curcumin on the apoptosis of cardiomyocytes and the expression of NF-κB, PPAR-gamma and Bcl-2 in rats with myocardial infarction injury. Exp. Ther. Med. 2016, 12, 3877–3884. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.C.; Ray, R.; Arya, D.S. Chrysin, a PPAR-gamma agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef]
- Cui, J.; Wang, G.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Neuroprotective effect of naringin, a flavone glycoside in quinolinic acid-induced neurotoxicity: Possible role of PPAR-gamma, Bax/Bcl-2, and caspase-3. Food Chem. Toxicol. 2018, 121, 95–108. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.A.; Hou, N.; Zhao, G.J.; Liu, X.W.; He, Y.Y.; Liu, H.L.; Hua, Y.Q.; Li, L.R.; Huang, Y.; Ou, C.W.; et al. Nrf2 Is a Key Regulator on Puerarin Preventing Cardiac Fibrosis and Upregulating Metabolic Enzymes UGT1A1 in Rats. Front. Pharmacol. 2018, 9, 540. [Google Scholar] [CrossRef]
- Ai, F.; Chen, M.; Yu, B.; Yang, Y.; Xu, G.; Gui, F.; Liu, Z.; Bai, X.; Chen, Z. Puerarin accelerate scardiac angiogenesis and improves cardiac function of myocardial infarction by upregulating VEGFA, Ang-1 and Ang-2 in rats. Int. J. Clin. Exp. Med. 2015, 8, 20821–20828. [Google Scholar] [PubMed]
- Cheng, W.; Wu, P.; Du, Y.; Wang, Y.; Zhou, N.; Ge, Y.; Yang, Z. Puerarin improves cardiac function through regulation of energy metabolism in Streptozotocin-Nicotinamide induced diabetic mice after myocardial infarction. Biochem. Biophys. Res. Commun. 2015, 463, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Rajadurai, M.; Stanely Mainzen Prince, P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology 2007, 230, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Geng, Q.; Yao, H.; Shen, Z.; Wu, Z.; Miao, X.; Shi, P. Protective effect of scutellarin on myocardial infarction induced by isoprenaline in rats. Iran. J. Basic. Med. Sci. 2018, 21, 267–276. [Google Scholar] [CrossRef]
- Gong, L.L.; Fang, L.H.; Wang, S.B.; Sun, J.L.; Qin, H.L.; Li, X.X.; Wang, S.B.; Du, G.H. Coptisine exert cardioprotective effect through anti-oxidative and inhibition of RhoA/Rho kinase pathway on isoproterenol-induced myocardial infarction in rats. Atherosclerosis 2012, 222, 50–58. [Google Scholar] [CrossRef]
- Iqbal, A.J.; McNeill, E.; Kapellos, T.S.; Regan-Komito, D.; Norman, S.; Burd, S.; Smart, N.; Machemer, D.E.; Stylianou, E.; McShane, H.; et al. Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 2014, 124, e33–e44. [Google Scholar] [CrossRef] [Green Version]
- Neri, T.; Armani, C.; Pegoli, A.; Cordazzo, C.; Carmazzi, Y.; Brunelleschi, S.; Bardelli, C.; Breschi, M.C.; Paggiaro, P.; Celi, A. Role of NF-κB and PPAR-gamma in lung inflammation induced by monocyte-derived microparticles. Eur. Respir. J. 2011, 37, 1494–1502. [Google Scholar] [CrossRef]
- Jiang, H. Literature analysis of 35 cases of acute hemolytic anemia due to puerarin. Tianjin Pharm. 2008, 20, 38–39. [Google Scholar]
- Zhou, Y.B.; Pan, W.S. The importance of pharmaceutical excipients according to the abnormal toxicity of puerarin. Chin. Med. Her. 2010, 7, 47–48. [Google Scholar]
- Wang, J.J.; Pahlm, O.; Warren, J.W.; Sapp, J.L.; Horacek, B.M. Criteria for ECG detection of acute myocardial ischemia: Sensitivity versus specificity. J. Electrocardiol. 2018, 51, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Reinstadler, S.J.; Baum, A.; Rommel, K.P.; Eitel, C.; Desch, S.; Mende, M.; Metzler, B.; Poess, J.; Thiele, H.; Eitel, I. ST-segment depression resolution predicts infarct size and reperfusion injury in ST-elevation myocardial infarction. Heart 2015, 101, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Mohler, P.J.; Schott, J.J.; Gramolini, A.O.; Dilly, K.W.; Guatimosim, S.; duBell, W.H.; Song, L.S.; Haurogne, K.; Kyndt, F.; Ali, M.E.; et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 2003, 421, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Aldosari, S.R.; Abid, M.R. Genetic Alterations in Oxidant and Anti-Oxidant Enzymes in the Vascular System. Front. Cardiovasc. Med. 2018, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.B.; Yuan, T.Y.; Wu, Y.J.; Yan, Y.; Li, L.; Xu, X.N.; Gong, L.L.; Qin, H.L.; Fang, L.H.; et al. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis 2013, 231, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.S.; Agarwal, H.; Barthwal, M.K. Cilostazol ameliorates heart failure with preserved ejection fraction and diastolic dysfunction in obese and non-obese hypertensive mice. J. Mol. Cell Cardiol. 2018, 123, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2014, 19, 681–694. [Google Scholar] [CrossRef]
- Ketsawatsomkron, P.; Sigmund, C.D. Molecular mechanisms regulating vascular tone by peroxisome proliferator activated receptor gamma. Curr. Opin. Nephrol. Hypertens. 2015, 24, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Y.; Zhang, T.; Guo, L.; Yang, W.; Zhang, J.; Wang, C. Role of Myoendothelial Gap Junctions in the Regulation of Human Coronary Artery Smooth Muscle Cell Differentiation by Laminar Shear Stress. Cell Physiol. Biochem. 2016, 39, 423–437. [Google Scholar] [CrossRef]
- Zhaocheng, J.; Jinfeng, L.; Luchang, Y.; Yequan, S.; Feng, L.; Kai, W. Ginkgolide A inhibits lipopolysaccharide-induced inflammatory response in human coronary artery endothelial cells via downregulation of TLR4-NF-κB signaling through PI3K/Akt pathway. Pharmazie 2016, 71, 588–591. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Wang, W.; Liu, Z.; Meng, J.; Han, Z. Mesenchymal Stem Cells Modified with Heme Oxygenase-1 Have Enhanced Paracrine Function and Attenuate Lipopolysaccharide-Induced Inflammatory and Oxidative Damage in Pulmonary Microvascular Endothelial Cells. Cell Physiol. Biochem. 2018, 49, 101–122. [Google Scholar] [CrossRef]
- Lin, G.; Shi, X.; Chen, S.; Lei, L.; You, X.; Huang, M.; Luo, L.; Li, Y.; Zhao, X.; Yan, F. Effects of micro-amounts of Porphyromonas gingivalis lipopolysaccharide on rabbit inflammatory immune response and development of atherosclerosis. J. Periodontal. Res. 2015, 50, 356–362. [Google Scholar] [CrossRef]
- Li, X.; Deroide, N.; Mallat, Z. The role of the inflammasome in cardiovascular diseases. J. Mol. Med. 2014, 92, 307–319. [Google Scholar] [CrossRef]
- Kang, P.M.; Izumo, S. Apoptosis in heart: Basic mechanisms and implications in cardiovascular diseases. Trends Mol. Med. 2003, 9, 177–182. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, Y.; Cheng, X.; Chen, J.; Chen, P.; Gao, X.; Zhang, Z. Myosin specific-T lymphocytes mediated myocardial inflammation in adoptive transferred rats. Cell Mol. Immunol. 2006, 3, 445–451. [Google Scholar] [CrossRef]
- Dang, X.; Du, G.; Hu, W.; Ma, L.; Wang, P.; Li, Y. Peroxisome proliferator-activated receptor gamma coactivator-1alpha/HSF1 axis effectively alleviates lipopolysaccharide-induced acute lung injury via suppressing oxidative stress and inflammatory response. J. Cell Biochem. 2018. [Google Scholar] [CrossRef]
- Hou, B.; Zhao, Y.; Qiang, G.; Yang, X.; Xu, C.; Chen, X.; Liu, C.; Wang, X.; Zhang, L.; Du, G. Puerarin Mitigates Diabetic Hepatic Steatosis and Fibrosis by Inhibiting TGF-beta Signaling Pathway Activation in Type 2 Diabetic Rats. Oxid. Med. Cell Longev. 2018, 2018, 4545321. [Google Scholar] [CrossRef]
- Tu, Y.M.; Gong, C.X.; Ding, L.; Liu, X.Z.; Li, T.; Hu, F.F.; Wang, S.; Xiong, C.P.; Liang, S.D.; Xu, H. A high concentration of fatty acids induces TNF-alpha as well as NO release mediated by the P2X4 receptor, and the protective effects of puerarin in RAW264.7 cells. Food Funct. 2017, 8, 4336–4346. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yuan, T.Y.; Zhang, H.F.; Wang, D.S.; Yan, Y.; Niu, Z.R.; Lin, Y.H.; Fang, L.H.; Du, G.H. Salvianolic acid A attenuates vascular remodeling in a pulmonary arterial hypertension rat model. Acta. Pharmacol. Sin. 2016, 37, 772–782. [Google Scholar] [CrossRef]
- Vorkapic, E.; Lundberg, A.M.; Mayranpaa, M.I.; Eriksson, P.; Wagsater, D. TRIF adaptor signaling is important in abdominal aortic aneurysm formation. Atherosclerosis 2015, 241, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |








| mRNA | Primer Sequence | |||
|---|---|---|---|---|
| hTNF-α 1 | F | 5′ | ATGAGCACTGAAAGCATGATC | 3′ |
| R | 5′ | TCACAGGGCAATGATCCCAAAGTAGACCTGCCC | 3′ | |
| hIL-6 2 | F | 5′ | GCCTTCGGTCCAGTTGCCTT | 3′ |
| R | 5′ | AGTGCCTCTTTGCTGCTTTCAC | 3′ | |
| hIL-1β | F | 5′ | CAGCCATGCCAGAAGTACCT | 3′ |
| R | 5′ | GACATCACCAAGCTTTTTTGC | 3′ | |
| hGAPDH | F | 5′ | CCACCCATGGCAAATTCCATGGCA | 3′ |
| R | 5′ | TCTAGACGGCAGGTCAGGTCCACC | 3′ | |
| mTNF-α | F | 5′ | GGCTGCCCCGACTACGT | 3′ |
| R | 5′ | AGGTTGACTTTCTCCTGGTATGAGA | 3′ | |
| mIL-6 | F | 5′ | TTCCATCCAGTTGCCTTCTTG | 3′ |
| R | 5′ | GGGAGTGGTATCCTCTGTGAAGTC | 3′ | |
| mIL-1β | F | 5′ | CTACAGGCTCCGAGATGAACAAC | 3′ |
| R | 5′ | TCCATTGAGGTGGAGAGCTTTC | 3′ | |
| mGAPDH 3 | F | 5′ | TGCACCACCAACTGCTTAGC | 3′ |
| R | 5′ | GGCATGGACTGTGGTCATGAG | 3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yuan, T.; Chen, D.; Chen, Y.; Sun, S.; Wang, D.; Fang, L.; Lu, Y.; Du, G. Cardioprotective Effects of Puerarin-V on Isoproterenol-Induced Myocardial Infarction Mice Is Associated with Regulation of PPAR-Υ/NF-κB Pathway. Molecules 2018, 23, 3322. https://doi.org/10.3390/molecules23123322
Li X, Yuan T, Chen D, Chen Y, Sun S, Wang D, Fang L, Lu Y, Du G. Cardioprotective Effects of Puerarin-V on Isoproterenol-Induced Myocardial Infarction Mice Is Associated with Regulation of PPAR-Υ/NF-κB Pathway. Molecules. 2018; 23(12):3322. https://doi.org/10.3390/molecules23123322
Chicago/Turabian StyleLi, Xuguang, Tianyi Yuan, Di Chen, Yucai Chen, Shuchan Sun, Danshu Wang, Lianhua Fang, Yang Lu, and Guanhua Du. 2018. "Cardioprotective Effects of Puerarin-V on Isoproterenol-Induced Myocardial Infarction Mice Is Associated with Regulation of PPAR-Υ/NF-κB Pathway" Molecules 23, no. 12: 3322. https://doi.org/10.3390/molecules23123322





