Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin
Abstract
:1. Introduction
1.1. Anticarcinogenic, Antioxidant and Anti-Inflammatory Properties of Curcumin
1.2. Curcumin and Alzheimer’s Disease
2. Computational Methods
2.1. Inverse Molecular Docking of Curcumin into Human Proteins
2.1.1. Proteome-Wide Binding Site Preparation
2.1.2. CANDOCK Docking Algorithm
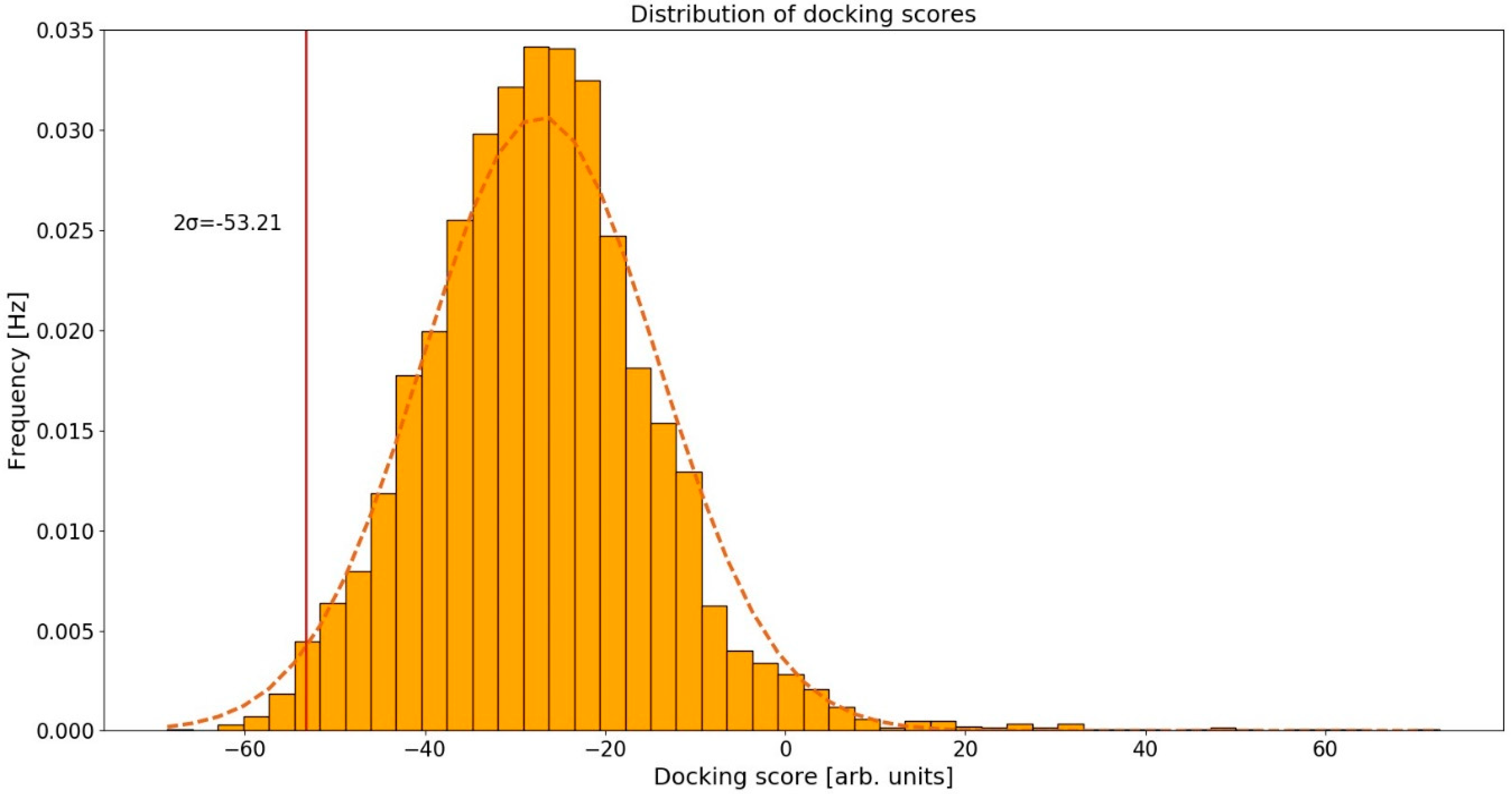
2.2. Distribution of Docking Scores
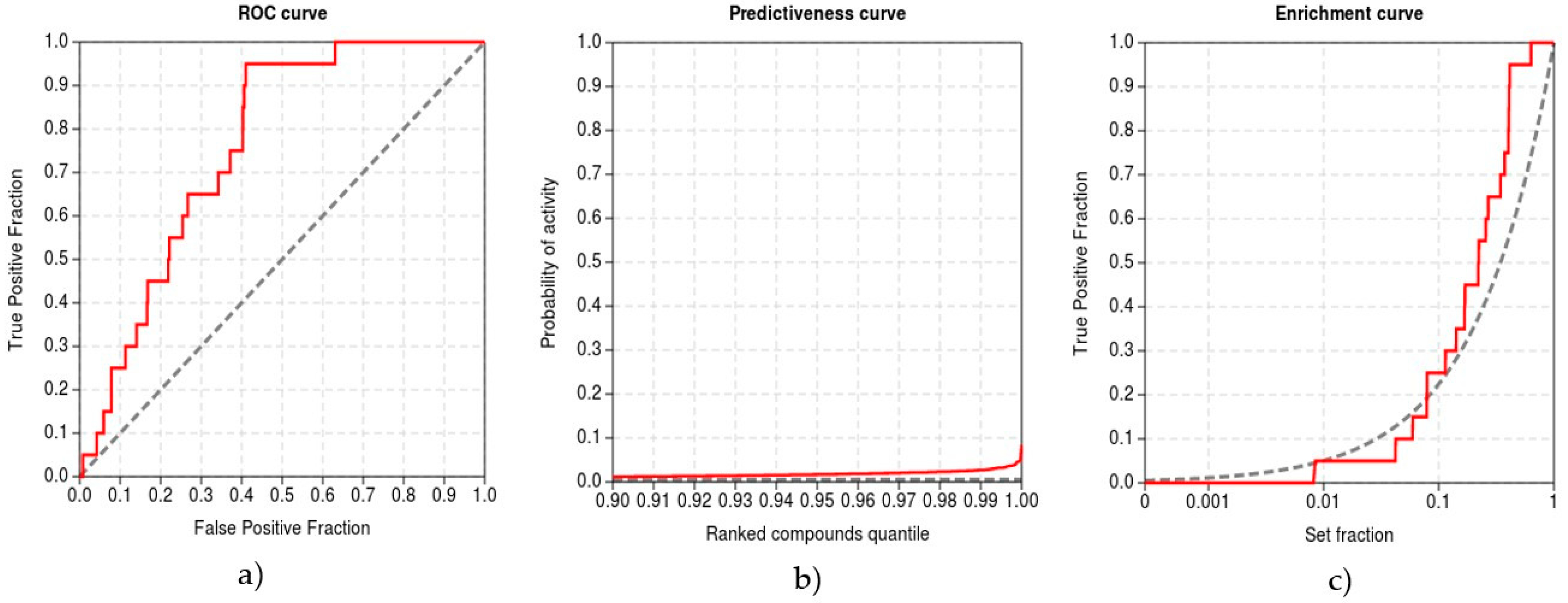
2.3. Validation of Inverse Docking Methodology
3. Results and Discussion
3.1. Identified Protein Targets
3.2. Anticarcinogenic Effects of Curcumin Explained by the Identified Protein Targets
3.3. Detailed Binding Poses of Curcumin in the Protein Targets FR-β and PDE4D with the Lowest Docking Score Values
3.4. Validation of the Inverse Molecular Docking Protocol
3.5. Critical Perspective
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABAD | amyloid β-peptide-binding alcohol dehydrogenase |
| AD | Alzheimer’s disease |
| AFB1 | aflatoxin B1 |
| AKR1C3 | aldo-keto reductase family 1 member C3 |
| AKT | protein kinase B (PKB) |
| AP-1 | activator protein 1 |
| ATF2 | activating transcription factor 2 |
| cAMP | cyclic adenosine monophosphate |
| COX | cyclooxygenase |
| CSF1 | the colony stimulating factor 1 |
| CSF1R | macrophage colony-stimulating factor 1 receptor |
| dCK | deoxycytidine kinase |
| DNA | deoxyribonucleic acid |
| e5NT | ecto-5’-nucleotidase |
| eNOS | endothelial nitric oxide synthase 3 |
| ERK | extracellular signal-regulated kinases |
| EP300 | histone acetyltransferase p300 |
| FADD | fas-associated protein with death domain |
| FR-β | human folate receptor β |
| HATs | histone acetyltransferases |
| HDACs | histone deacetylases |
| HSD10 | 17-β-hydroxysteroid dehydrogenase type 10 |
| IL-1 | interleukin 1 |
| IκB | inhibitory protein kappa B |
| MMPs | matrix metalloproteinases |
| MAPK | mitogen-activated protein kinases |
| MEK | mitogen-activated protein kinase kinase |
| NF-κB | nuclear factor κB |
| NMR | nuclear magnetic resonance |
| nr-PDB | non-redundant Protein Data Bank |
| PKC | protein kinase C |
| PDB | Protein Data Bank |
| PDEs | cyclic nucleotide phosphodiesterase enzymes |
| PI3 | phosphatidylinositide 3-kinase |
| PLCγ | phospholipase C gamma |
| RAF | rapidly accelerated fibrosarcoma |
| ROS | reactive oxygen species |
| STAT | signal transducer and activator of transcription protein |
| TNF-α | tumor necrosis factor α |
| TP53 | tumor protein p53 |
| tRNA | transport ribonucleic acid |
| Wnt | wingless-related integration site |
References
- Klebe, G. Virtual ligand screening: Strategies, perspectives and limitations. Drug Discov. Today 2006, 11, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Tame, J.R. Scoring functions—The first 100 years. J. Comput. Aided Mol. Des. 2005, 19, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pfalzer, A.C.; Koh, G.Y.; Tang, S.; Crott, J.W.; Thomas, M.J.; Meydani, M.; Mason, J.B. Curcumin and Salsalate Suppresses Colonic Inflammation and Procarcinogenic Signaling in High-Fat-Fed, Azoxymethane-Treated Mice. J. Agric. Food. Chem. 2017, 65, 7200–7209. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Chen, Y.C.; Huang, Y.T.; Lin-Shiau, S.Y. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J. Cell. Biochem. 1997, 67, 39–48. [Google Scholar] [CrossRef]
- Marcu, M.G.; Jung, Y.-J.; Lee, S.; Chung, E.-J.; Lee, M.-J.; Trepel, J.; Neckers, L. Curcumin is an inhibitor of p300 histone acetylatransferase. J. Med. Chem. 2006, 2, 169–174. [Google Scholar]
- Lee, S.J.; Krauthauser, C.; Maduskuie, V.; Fawcett, P.T.; Olson, J.M.; Rajasekaran, S.A. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer 2011, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Sonkaew, P.; Sane, A.; Suppakul, P. Antioxidant activities of curcumin and ascorbyl dipalmitate nanoparticles and their activities after incorporation into cellulose-based packaging films. J. Agric. Food. Chem. 2012, 60, 5388–5399. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stępkowski, T.M.; Brzóska, K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Butterfield, D.; Stella, A. Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: Novel targets for neuroprotection in Alzheimer’s disease. Ital. J. Biochem. 2003, 52, 177–181. [Google Scholar] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]
- Perry, M.C.; Demeule, M.; Regina, A.; Moumdjian, R.; Beliveau, R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010, 54, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Palma, G.; Luciano, A.; Rea, D.; Arra, C. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. BioMed Res. Int. 2013, 2013, 810423. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Lin, Y.-W.; Chiu, H.-M.; Chiang, B.-H. Curcumin induces apoptosis of colorectal cancer stem cells by coupling with CD44 marker. J. Agric. Food Chem. 2016, 64, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.C.P.; Lokesh, B.R. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol. Cell. Biochem. 1994, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, M.; Rao, M. Curcumin inhibits nitrogen dioxide induced oxidation of hemoglobin. Mol. Cell. Biochem. 1995, 146, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006, 79, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Divya, C.S.; Pillai, M.R. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol. Carcinog. 2006, 45, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.-Y. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharmacol. 1995, 49, 1551–1556. [Google Scholar] [CrossRef]
- Rashmi, R.; Santhosh Kumar, T.; Karunagaran, D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett. 2003, 538, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. β-Catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhuri, T.; Pal, S.; Das, T.; Sa, G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J. Biol. Chem. 2005, 280, 20059–20068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Okan, I.; Szekely, L.; Klein, G.; Wiman, K.G. bcl-2 Inhibits Wild-Type p53-triggered Apoptosis but not G~ 1 Cell Cycle Arrest and Transactivation of WAF1 and bax. Cell Growth Differ. 1995, 6, 1071–1076. [Google Scholar] [PubMed]
- Kim, J.Y.; Cho, T.J.; Woo, B.H.; Choi, K.U.; Lee, C.H.; Ryu, M.H.; Park, H.R. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch. Oral Biol. 2012, 57, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Nerlich, A.G.; Iancu, C.M.; Cilli, M.; Schleicher, E.; Vené, R.; Dell’Eva, R.; Jochum, M.; Albini, A.; Pfeffer, U. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell. Physiol. Biochem. 2007, 19, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer9s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, L.; Pan, X.; Chen, J.; Wang, L.; Wang, W.; Cheng, R.; Wu, F.; Feng, X.; Yu, Y. The effect of resveratrol on β amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget 2016, 7, 17380. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.A.; Konc, J.; Samudrala, R.; Chopra, G. CANDOCK: Chemical atomic network based hierarchical flexible docking algorithm using generalized statistical potentials. bioRxiv 2018. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S. The protein data bank. Acta Crystallogr. Sect. D. Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Štular, T.; Lesnik, S.; Rozman, K.; Schink, J.; Zdouc, M.; Ghysels, A.; Liu, F.; Aldrich, C.C.; Haupt, V.J.; Salentin, S.; et al. Discovery of mycobacterium tuberculosis InhA inhibitors by binding sites comparison and ligands prediction. J. Med. Chem. 2016, 59, 11069–11078. [Google Scholar] [CrossRef] [PubMed]
- Konc, J.; Janežič, D. ProBiS-2012: Web server and web services for detection of structurally similar binding sites in proteins. Nucleic Acids Res. 2012, 40, W214–W221. [Google Scholar] [CrossRef] [PubMed]
- Konc, J.; Janezic, D. An improved branch and bound algorithm for the maximum clique problem. Match Commun. Math. Comput. Chem. 2007, 58, 569–590. [Google Scholar]
- Edeas, M.; Khalfoun, Y.; Lazizi, Y.; Vergnes, L.; Labidalle, S.; Postaire, E.; Lindenbaum, A. Effect of the liposolubility of free radical scavengers on the production of antigen P24 from a HIV infected monocytic cell line. C. R. Seances Soc. Biol. Fil. 1995, 189, 367–373. [Google Scholar] [PubMed]
- Alvarez, J.C. High-throughput docking as a source of novel drug leads. Curr. Opin. Chem. Biol. 2004, 8, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Triballeau, N.; Acher, F.; Brabet, I.; Pin, J.-P.; Bertrand, H.-O. Virtual screening workflow development guided by the “receiver operating characteristic” curve approach. Application to high-throughput docking on metabotropic glutamate receptor subtype 4. J. Med. Chem. 2005, 48, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Truchon, J.-F.; Bayly, C.I. Evaluating virtual screening methods: Good and bad metrics for the “early recognition” problem. J. Chem. Inf. Model. 2007, 47, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Empereur-mot, C.; Guillemain, H.; Latouche, A.; Zagury, J.-F.; Viallon, V.; Montes, M. Predictiveness curves in virtual screening. J. Cheminform. 2015, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.P.; Singh, S.B.; Fluder, E.M.; Kearsley, S.K. Protocols for bridging the peptide to nonpeptide gap in topological similarity searches. J. Chem. Inf. Comput. Sci. 2001, 41, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Empereur-Mot, C.; Zagury, J.-F.; Montes, M. Screening explorer—An interactive tool for the analysis of screening results. J. Chem. Inf. Model. 2016, 56, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.S.; Singh, M.; Reeder, K.M.; Carter, J.J.; Kovach, A.R.; Meng, W.; Ratnam, M.; Zhang, F.; Dann, C.E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. USA. 2013, 110, 15180–15188. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.; Biswal, B.K.; Sumantran, V.N.; Verma, R.S. Augmented sensitivity to methotrexate by curcumin induced overexpression of folate receptor in KG-1 cells. Biochimie 2013, 95, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.B.; Magnusson, O.T.; Singh, J.; Witte, P.; Staker, B.L.; Bjornsson, J.M.; Thorsteinsdottir, M.; Hrafnsdottir, S.; Hagen, T.; Kiselyov, A.S. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010, 28, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Abusnina, A.; Keravis, T.; Zhou, Q.; Justiniano, H.; Lobstein, A.; Lugnier, C. Tumour growth inhibition and anti-angiogenic effects using curcumin correspond to combined PDE2 and PDE4 inhibition. Thromb. Haemost. 2015, 114, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Du Yan, S.; Fu, J.; Soto, C.; Chen, X.; Zhu, H.; Al-Mohanna, F.; Collison, K.; Zhu, A.; Stern, E.; Saido, T. An intracellular protein that binds amyloid-β peptide and mediates neurotoxicity in Alzheimer’s disease. Nature 1997, 389, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; He, X.-Y.; Isaacs, C.; Dobkin, C.; Miller, D.; Philipp, M. Roles of 17β-hydroxysteroid dehydrogenase type 10 in neurodegenerative disorders. J. Steroid Biochem. Mol. Biol. 2014, 143, 460–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, D.; Jurkowska, R.Z.; Zhang, X.; Jeltsch, A.; Cheng, X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature 2007, 449, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Sadeghizadeh, M.; Behmanesh, M.; Najafi, F. Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine 2015, 22, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Morgunova, E.; Tuuttila, A.; Bergmann, U.; Isupov, M.; Lindqvist, Y.; Schneider, G.; Tryggvason, K. Structure of human pro-matrix metalloproteinase-2: Activation mechanism revealed. Science 1999, 284, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-S.; Lai, K.-C.; Hsu, S.-C.; Yang, J.-S.; Kuo, C.-L.; Lin, J.-P.; Ma, Y.-S.; Wu, C.-C.; Chung, J.-G. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and-9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett. 2009, 285, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-C.; Chen, G.-W.; Lin, J.-G.; WU, L.-T.; CHUNG, J.-G. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B/p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006, 26, 1281–1288. [Google Scholar] [PubMed]
- Sabini, E.; Hazra, S.; Ort, S.; Konrad, M.; Lavie, A. Structural basis for substrate promiscuity of dCK. J. Mol. Biol. 2008, 378, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Moniot, S.; Schutkowski, M.; Steegborn, C. Crystal structure analysis of human Sirt2 and its ADP-ribose complex. J. Struct. Biol. 2013, 182, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Wang, S.M.; Villaseñor, A.G.; Tsing, S.; Walter, D.; Browner, M.F.; Barnett, J.; Kuglstatter, A. The crystal structure of JNK2 reveals conformational flexibility in the MAP kinase insert and indicates its involvement in the regulation of catalytic activity. J. Mol. Biol. 2008, 383, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Tan, T.-H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 1998, 17, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zheng, W.; Xin, N.; Chi, Z.-H.; Wang, N.-Q.; Nie, Y.-X.; Feng, W.-Y.; Wang, Z.-Y. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res. 2010, 13, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Knapp, K.; Zebisch, M.; Pippel, J.; El-Tayeb, A.; Müller, C.E.; Sträter, N. Crystal structure of the human ecto-5′-nucleotidase (CD73): Insights into the regulation of purinergic signaling. Structure 2012, 20, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Pannone, L.; Bocchinfuso, G.; Flex, E.; Rossi, C.; Baldassarre, G.; Lissewski, C.; Pantaleoni, F.; Consoli, F.; Lepri, F.; Magliozzi, M. Structural, Functional, and Clinical Characterization of a Novel PTPN11 Mutation Cluster Underlying Noonan Syndrome. Hum. Mutat. 2017, 38, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Angelov, D.; Molla, A.; Perche, P.-Y.; Hans, F.; Côté, J.; Khochbin, S.; Bouvet, P.; Dimitrov, S. The histone variant macroH2A interferes with transcription factor binding and SWI/SNF nucleosome remodeling. Mol. Cell 2003, 11, 1033–1041. [Google Scholar] [CrossRef]
- Doyen, C.-M.; An, W.; Angelov, D.; Bondarenko, V.; Mietton, F.; Studitsky, V.M.; Hamiche, A.; Roeder, R.G.; Bouvet, P.; Dimitrov, S. Mechanism of polymerase II transcription repression by the histone variant macroH2A. Mol. Cell. Biol. 2006, 26, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Juhn, K.; Lee, H.; Kim, S.-H.; Min, B.-H.; Lee, K.-M.; Cho, M.-H.; Park, G.-H.; Lee, K.-H. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp. Mol. Med. 2007, 39, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, D.; Zhao, Y.; Tu, B.; Zheng, Z.; Wang, L.; Wang, H.; Gu, W.; Roeder, R.G.; Zhu, W.-G. Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1). Proc. Natl. Acad. Sci. USA. 2011, 108, 1925–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullrich, A.; Coussens, L.; Hayflick, J.S.; Dull, T.J.; Gray, A.; Tam, A.; Lee, J.; Yarden, Y.; Libermann, T.A.; Schlessinger, J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984, 309, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol. Urol. 2000, 4, 1–6. [Google Scholar] [PubMed]
- Chen, A.; Xu, J.; Johnson, A. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 2006, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-O.; Jang, Y.-S.; Heo, J.-S.; Chung, W.-T.; Choi, K.-C.; Lee, J.-C. Apoptosis-inducing factor plays a critical role in caspase-independent, pyknotic cell death in hydrogen peroxide-exposed cells. Apoptosis 2009, 14, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small molecule inhibitors targeting activator protein 1 (AP-1) miniperspective. J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef] [PubMed]
- Patsialou, A.; Wyckoff, J.; Wang, Y.; Goswami, S.; Stanley, E.R.; Condeelis, J.S. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009, 69, 9498–9506. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Stanley, E.R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 2006, 18, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gripp, K.W.; Bifeld, E.; Stabley, D.L.; Hopkins, E.; Meien, S.; Vinette, K.; Sol-Church, K.; Rosenberger, G. A novel HRAS substitution (c. 266C> G; p. S89C) resulting in decreased downstream signaling suggests a new dimension of RAS pathway dysregulation in human development. Am. J. Med. Genet. A 2012, 158, 2106–2118. [Google Scholar] [CrossRef] [PubMed]
- Bodreddigari, S.; Jones, L.K.; Egner, P.A.; Sutter, C.H.; Roebuck, B.D.; Guengerich, F.P.; Kensler, T.W.; Sutter, T.R. Protection against aflatoxin B1-induced cytotoxicity by expression of the cloned aflatoxin B1-aldehyde reductases rat AKR7A1 and human AKR7A3. Chem. Res. Toxicol. 2008, 21, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.L.; Ride, J.P.; Bunce, C.M.; Desmond, J.C.; Cummings, S.M.; White, S.A. Crystal structures of prostaglandin D2 11-ketoreductase (AKR1C3) in complex with the nonsteroidal anti-inflammatory drugs flufenamic acid and indomethacin. Cancer Res. 2004, 64, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- McGrath, A.P.; Hilmer, K.M.; Collyer, C.A.; Shepard, E.M.; Elmore, B.O.; Brown, D.E.; Dooley, D.M.; Guss, J.M. Structure and inhibition of human diamine oxidase. Biochemistry 2009, 48, 9810–9822. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Huart, A.-S.; Temmerman, K.; Vahokoski, J.; Mertens, H.D.; Komadina, D.; Hoffmann, J.-E.; Yumerefendi, H.; Svergun, D.I.; Kursula, P. Death-Associated Protein Kinase Activity Is Regulated by Coupled Calcium/Calmodulin Binding to Two Distinct Sites. Structure 2016, 24, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, K.; Slike, B.M.; Hood, J.; Otani, A.; Ewalt, K.L.; Friedlander, M.; Cheresh, D.A.; Schimmel, P. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc. Natl. Acad. Sci. USA. 2002, 99, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Atzeri, A.; Nieddu, M.; Appendino, G. New insights into the antioxidant activity and cytotoxicity of arzanol and effect of methylation on its biological properties. Chem. Phys. Lipids 2017, 205, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrián-Uhalte, E. The ChEMBL database in 2017. Nucleic Acids Res. 2016, 45, D945–D954. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.C. Folate receptors. Annu. Rev. Nutr. 1996, 16, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Gonen, N.; Assaraf, Y.G. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resist. Updat. 2012, 15, 183–210. [Google Scholar] [CrossRef] [PubMed]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar] [PubMed]
- Puig-Kröger, A.; Sierra-Filardi, E.; Domínguez-Soto, A.; Samaniego, R.; Corcuera, M.T.; Gómez-Aguado, F.; Ratnam, M.; Sánchez-Mateos, P.; Corbí, A.L. Folate receptor β is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009, 69, 9395–9403. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.A.; Teteloshvili, N.; Zeebregts, C.J.; Westra, J.; Bijl, M. Macrophage folate receptor-β (FR-β) expression in auto-immune inflammatory rheumatic diseases: A forthcoming marker for cardiovascular risk? Autoimmun. Rev. 2012, 11, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.F.; Wang, H.; Behm, F.G.; Mathew, P.; Wu, M.; Booth, R.; Ratnam, M. Folate receptor type β is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer 1999, 85, 348–357. [Google Scholar] [CrossRef]
- Bondì, M.L.; Emma, M.R.; Botto, C.; Augello, G.; Azzolina, A.; Di Gaudio, F.; Craparo, E.F.; Cavallaro, G.; Bachvarov, D.; Cervello, M. Biocompatible lipid nanoparticles as carriers to improve curcumin efficacy in ovarian cancer treatment. J. Agric. Food. Chem. 2017, 65, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Improved Chemical Stability and Antiproliferative Activities of Curcumin-Loaded Nanoparticles with a Chitosan Chlorogenic Acid Conjugate. J. Agric. Food. Chem. 2017, 65, 10812–10819. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.H.; Chung, S.K.; Kim, J.T.; Joung, H.J.; Park, H.J. Preparation of chitosan-coated nanoliposomes for improving the mucoadhesive property of curcumin using the ethanol injection method. J. Agric. Food. Chem. 2013, 61, 11119–11126. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, Y.-H.; Cheng, Y.-K.; Lu, X.; Shao, Y.-X.; Li, X.; Liu, M.; Liu, P.; Luo, H.-B. Identification of novel phosphodiesterase-4D inhibitors prescreened by molecular dynamics-augmented modeling and validated by bioassay. J. Chem. Inf. Model. 2013, 53, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Yan, G.-H.; Chai, O.H.; Song, C.H. Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation. Anat. Cell Biol. 2010, 43, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Akiri, O.F.; Durojaiye, M.A.; Alabi, A.F. Combined administration of curcumin and gallic acid inhibits gallic acid-induced suppression of steroidogenesis, sperm output, antioxidant defenses and inflammatory responsive genes. J. Steroid Biochem. Mol. Biol. 2014, 143, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Konc, J.; Miller, B.-T.; Štular, T.; Lešnik, S.; Woodcock, H.-L.; Brooks, B.-R.; Janežič, D. ProBiS-CHARMMing: Web Interface for Prediction and Optimization of Ligands in Protein Binding Sites. J. Chem. Inf. Model. 2015, 55, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.M.; Bren, U.; Haltrich, D.; Oostenbrink, C. Molecular dynamics simulations give insight into D-glucose dioxidation at C2 and C3 by Agaricus meleagris pyranose dehydrogenase. J. Comput. Aided Mol. Des. 2013, 27, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Uyar, T. Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: Enhanced water solubility and slow release of curcumin. Int. J. Pharm. 2017, 518, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iranshahi, M.; Chini, M.G.; Masullo, M.; Sahebkar, A.; Javidnia, A.; Chitsazian Yazdi, M.; Pergola, C.; Koeberle, A.; Werz, O.; Pizza, C. Can small chemical modifications of natural pan-inhibitors modulate the biological selectivity? The case of curcumin prenylated derivatives acting as HDAC or mPGES-1 inhibitors. J. Nat. Prod. 2015, 78, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Yogosawa, S.; Yamada, Y.; Yasuda, S.; Sun, Q.; Takizawa, K.; Sakai, T. Dehydrozingerone, a structural analogue of curcumin, induces cell-cycle arrest at the G2/M phase and accumulates intracellular ROS in HT-29 human colon cancer cells. J. Nat. Prod. 2012, 75, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| PDB ID with Chain | Protein Name | Predicted Docking Scores (arb. Units) | Protein Function and Reported Connection with Diseases | Reported Experimental Correlation with Curcumin * |
|---|---|---|---|---|
| 4kmyA | human folate receptor β (FR-β) | −63.30 | A target for the specific delivery of antifolates or folate conjugates to tumors or sites of inflammation [49]. | Yes [50] |
| 3iadA | cAMP-specific 3′,5′-cyclic phosphodiesterase 4D (PDE4D) | −62.24 | Modulation of cAMP signaling, important in the treatment of Alzheimer’s disease, Huntington’s disease, schizophrenia, and depression [51]. | Yes [52] |
| 1u7tA | 17-β-hydroxysteroid dehydrogenase type 10 (17β-HSD10) | −61.46 | Interacts with amyloid-β, connection with neuronal dysfunction associated with Alzheimer’s disease [53,54]. | No |
| 2qrvA | DNA (cytosine-5)-methyltransferase 3A | −58.59 | Required for genome-wide de novo methylation of DNA. Represses transcription through HDAC [55]. | Yes [56] |
| 1ck7A | metalloproteinase-2 (MMP-2) | −57.93 | Involved in angiogenesis, tissue repair, tumor invasion and inflammation. Initiates a primary innate immune response with the activation of the NF-κB transcriptional pathway [57,58]. | Yes [59] |
| 3qeoA | deoxycytidine kinase (dCK) | −57.37 | Required for the phosphorylation of deoxyribonucleosides and nucleoside analogs in antiviral and chemotherapeutic agents [60]. | No |
| 4x3oA | NAD-dependent protein deacetylase sirtuin-2 | −56.96 | Involved in the cell cycle, genomic integrity, microtubule dynamics, cell differentiation, metabolic networks, and autophagy. Deacetylates RELA in the cytoplasm inhibiting NF-κB-dependent transcription activation upon TNF-α stimulation [61]. | No |
| 3e7oA | mitogen-activated protein kinase 9 (MAPK-9) | −56.93 | Regulates cell proliferation, differentiation, migration and programmed cell death. Phosphorylates AP-1 components c-Jun and ATF2 and thus regulates AP-1 transcriptional activity. Promotes β-catenin/CTNNB1 degradation and inhibits the Wnt signaling pathway [62,63]. | Yes [64] |
| 4h2iA | ecto-5′-nucleotidase (e5NT) | −55.95 | Activates P1 adenosine receptors, and has emerged as a drug target for treatment of inflammation, chronic pain, hypoxia, and cancer [65]. | No |
| 4nwgA | tyrosine-protein phosphatase non-receptor type 11 | −55.49 | Positively regulates the MAPK signal transduction pathway [66]. | No |
| 1zr3A | core histone macro-H2A.1 | −55.46 | Inhibits histone acetylation by EP300, recruits class I HDACs, which represses transcription. Inhibits the binding of transcription factor NF-κB [67,68]. | No |
| 4zzjA | NAD-dependent protein deacetylase sirtuin-1 | −54.89 | Coordinates the cell cycle, response to DNA damage, metabolism, apoptosis, deacetylation of histones and autophagy. Deacetylates ‘Lys-382’ of p53/TP53 as well as RELA/NF-κB p65 and impairs its ability to induce apoptosis. Modulates AP-1 transcription factor activity [69,70,71]. | No |
| 4zseA | epidermal growth factor receptor | −54.81 | Activates major downstream signaling cascades Ras-RAF-MEK-ERK, PI3 kinase-AKT, PLCγ-PKC, STATs modules and NF-κB. [72,73] | Yes [74] |
| 5kviA | apoptosis-inducing factor 1 (AP-1) | −54.76 | NADH oxidoreductase and a regulator of apoptosis in a caspase-independent pathway [75]. | Yes [76] |
| 3lcoA | macrophage colony-stimulating factor 1 receptor (CSF1R) | −54.59 | Regulates proliferation and differentiation of macrophages and monocytes. Promotes the release of proinflammatory chemokines in response to IL34 and CSF1. Mediates activation of the MAPK1/ERK2 and/or MAPK3/ERK1 [77,78]. | No |
| 2rgcA | GTPase HRas | −54.43 | Activation of Ras signal transduction pathway [79]. | No |
| 2clpA | aflatoxin B1 aldehyde reductase member 3 | −53.86 | Reduces the dialdehyde protein-binding form of aflatoxin B1 (AFB1) to the non-binding AFB1 dialcohol. Involved in the protection of the liver against the toxic and carcinogenic effects of AFB1 [80]. | No |
| 1s1pA | aldo-keto reductase family 1 member C3 (AKR1C3) | −53.69 | Suppresses cell differentiation and promotes proliferation in myeloid cells. Possesses potential in new anticancer therapies with reduced COX-dependent side effects [81]. | No |
| 3hi7A | amiloride-sensitive amine oxidase | −53.51 | Catalyzes cell proliferation, tissue differentiation, tumor formation, and possibly apoptosis [82]. | No |
| 2a2aA | death-associated protein kinase 2 | −53.41 | Triggers cell survival, apoptosis, and autophagy. Regulates type I apoptotic and type II autophagic cell death signals, depending on the cellular setting [83] | No |
| 1r6tA | tryptophan-tRNA ligase | −53.31 | Regulates ERK, AKT (PKB), and eNOS activation pathways associated with angiogenesis [84]. | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, V.; Konc, J.; Bren, U. Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin. Molecules 2018, 23, 3351. https://doi.org/10.3390/molecules23123351
Furlan V, Konc J, Bren U. Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin. Molecules. 2018; 23(12):3351. https://doi.org/10.3390/molecules23123351
Chicago/Turabian StyleFurlan, Veronika, Janez Konc, and Urban Bren. 2018. "Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin" Molecules 23, no. 12: 3351. https://doi.org/10.3390/molecules23123351







