Coordination-Enhanced Luminescence on Tetra-Phenylethylene-Based Supramolecular Assemblies
Abstract
:1. Introduction
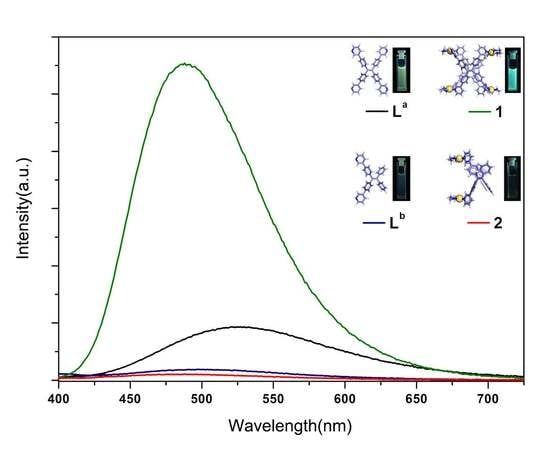
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis Procedure
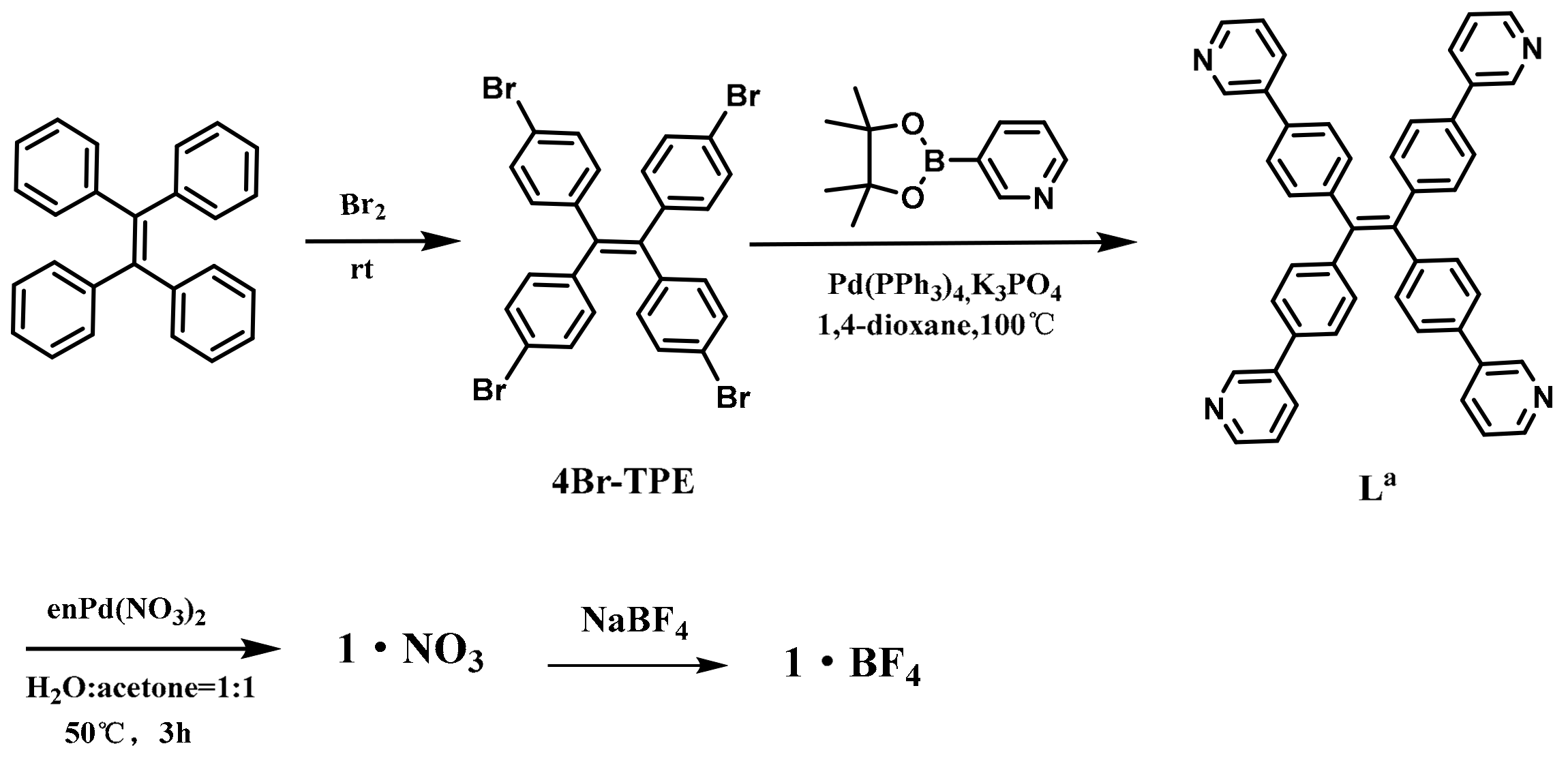
3.2.1. Synthesis of Ligand La
3.2.2. Synthesis of Assembly 1
3.2.3. Synthesis of Ligand Lb and Assembly 2
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 0, 1740–1741. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Shustova, N.B.; McCarthy, B.D.; Dincă, M. Turn-On Fluorescence in Tetraphenylethylene-Based Metal–Organic Frameworks: An Alternative to Aggregation-Induced Emission. J. Am. Chem. Soc. 2011, 133, 20126–20129. [Google Scholar] [CrossRef] [PubMed]
- Shustova, N.B.; Ong, T.C.; Cozzolino, A.F.; Michaelis, V.K.; Griffin, R.G.; Dinca, M. Phenyl ring dynamics in a tetraphenylethylene-bridged metal-organic framework: Implications for the mechanism of aggregation-induced emission. J. Am. Chem. Soc. 2012, 134, 15061–15070. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Q.; Zhang, X.; Nie, C.B.; Pang, Z.F.; Xu, X.N.; Zhao, X. The construction of a two-dimensional supramolecular organic framework with parallelogram pores and stepwise fluorescence enhancement. Chem. Commun. 2015, 51, 16417–16420. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, K.; Li, X.; Qian, Y.; Zhu, H.; Yuan, D.; Xu, Q.H.; Jiang, J.; Zhao, D. Ultrathin two-dimensional porous organic nanosheets with molecular rotors for chemical sensing. Nat. Commun. 2017, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lam, J.W.Y.; Tang, B.Z. Tetraphenylethene: A versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 2012, 22, 23726–23740. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, D.; Zhao, Y.; Yang, X.J.; Wang, Y.Y.; Wu, B. Anion-coordination-induced turn-on fluorescence of an oligourea-functionalized tetraphenylethene in a wide concentration range. Angew. Chem. Int. Ed. Engl. 2014, 53, 6632–6636. [Google Scholar] [CrossRef] [PubMed]
- August, D.P.; Nichol, G.S.; Lusby, P.J. Maximizing Coordination Capsule–Guest Polar Interactions in Apolar Solvents Reveals Significant Binding. Angew. Chem. 2016, 128, 15246–15250. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, Z.Y.; Arvapally, R.K.; Chen, Y.P.; McDougald, R.N., Jr.; Ivy, J.F.; Yakovenko, A.A.; Feng, D.; Omary, M.A.; Zhou, H.C. Rigidifying fluorescent linkers by metal-organic framework formation for fluorescence blue shift and quantum yield enhancement. J. Am. Chem. Soc. 2014, 136, 8269–8276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Su, J.; Feng, D.; Wei, Z.; Zou, X.; Zhou, H. Piezofluorochromic Metal-Organic Framework: A Micro-Scissor Lift. J. Am. Chem. Soc. 2015, 137, 10064–10067. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, M.; Cook, T.R.; Zhang, M.; Saha, M.L.; Zhou, Z.; Li, X.; Huang, F.; Stang, P.J. Light-Emitting Superstructures with Anion Effect: Coordination-Driven Self-Assembly of Pure Tetraphenylethylene Metallacycles and Metallacages. J. Am. Chem. Soc. 2016, 138, 4580–4588. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yao, L.; Zhang, J.; Mu, Y.; Chi, Z.; Su, C.-Y. A luminescent silver-phosphine tetragonal cage based on tetraphenylethylene. Dalton Trans. 2016, 45, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-X.; Chen, L.-J.; Yang, H.-B. Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly: From structure to functions. Chem. Soc. Rev. 2015, 44, 2148–2167. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cook, T.R.; Wang, P.; Huang, F.; Stang, P.J. Highly emissive platinum(II) metallacages. Nat. Chem. 2015, 7, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, H.; Hauke, C.E.; Cook, T.R.; Wang, M.; Saha, M.L.; Zhou, Z.; Zhang, M.; Li, X.; Huang, F.; et al. A Suite of Tetraphenylethylene-Based Discrete Organoplatinum(II) Metallacycles: Controllable Structure and Stoichiometry, Aggregation-Induced Emission, and Nitroaromatics Sensing. J. Am. Chem. Soc. 2015, 137, 15276–15286. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Ren, Y.-Y.; Wu, N.-W.; Sun, B.; Ma, J.-Q.; Zhang, L.; Tan, H.; Liu, M.; Li, X.; Yang, H.-B. Hierarchical Self-Assembly of Discrete Organoplatinum(II) Metallacycles with Polysaccharide via Electrostatic Interactions and Their Application for Heparin Detection. J. Am. Chem. Soc. 2015, 137, 11725–11735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Tan, L.; Zhai, T.-L.; Wang, S.; Tan, B.; Zheng, Y.-S.; Yang, X.-L.; Xu, H.-B. A Porous Tricyclooxacalixarene Cage Based on Tetraphenylethylene. Angew. Chem. Int. Ed. 2015, 54, 9244–9248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, G.L.; Yan, Q.Q.; Zhou, L.P.; Cai, L.X.; Guo, X.Q.; Sun, Q.F. Self-Assembly of a Tetraphenylethylene-Based Capsule Showing Both Aggregation- and Encapsulation-Induced Emission Properties. Inorg. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Kumar, A.; Howlader, P.; Mukherjee, P.S. A Self-Assembled Trigonal Prismatic Molecular Vessel for Catalytic Dehydration Reactions in Water. Chem. Eur. J. 2017, 23, 12565–12574. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizzi, L.; Licchelli, M.; Pallavicini, P.; Perotti, A.; Taglietti, A.; Sacchi, D. Fluorescent Sensors for Transition Metals Based on Electron-Transfer and Energy-Transfer Mechanisms. Chem. Eur. J. 1996, 2, 75–82. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Peng, X. Colourimetric and fluorescent probes for the optical detection of palladium ions. Chem. Soc. Rev. 2013, 42, 7943–7962. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xu, Y.; Qian, X. Highly sensitive and selective Pd2+ sensor of naphthalimide derivative based on complexation with alkynes and thio-heterocycle. Chem. Commun. 2008, 6339–6341. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Laschat, S.; Diele, S.; Nimtz, M. Tetraphenylethene-Derived Columnar Liquid Crystals and Their Oxidative Photocyclization. Eur. J. Org. Chem. 2003, 2829–2839. [Google Scholar] [CrossRef]
- De, S.; Mahata, K.; Schmittel, M. Metal-coordination-driven dynamic heteroleptic architectures. Chem. Soc. Rev. 2010, 39, 1555–1575. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, P.P.; Widen, J.C.; Magnus, M.A.; Swenson, D.C.; Pigge, F.C. Tetrapyridyl tetraphenylethylenes: Supramolecular building blocks with aggregation-induced emission properties. Tetrahedron Lett. 2011, 52, 2519–2522. [Google Scholar] [CrossRef]
- Cohen, Y.; Avram, L.; Frish, L. Diffusion NMR Spectroscopy in Supramolecular and Combinatorial Chemistry: An Old Parameter—New Insights. Angew. Chem. Int. Ed. 2005, 44, 520–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.-P.; Chao, H.-Y.; Yuan, D.; Tan, T.T.Y.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-Dimensional Metal–Organic Framework with Wide Channels and Responsive Turn-On Fluorescence for the Chemical Sensing of Volatile Organic Compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.-Q.; Hu, S.-J.; Zhang, G.-L.; Zhang, T.; Zhou, L.-P.; Sun, Q.-F. Coordination-Enhanced Luminescence on Tetra-Phenylethylene-Based Supramolecular Assemblies. Molecules 2018, 23, 363. https://doi.org/10.3390/molecules23020363
Yan Q-Q, Hu S-J, Zhang G-L, Zhang T, Zhou L-P, Sun Q-F. Coordination-Enhanced Luminescence on Tetra-Phenylethylene-Based Supramolecular Assemblies. Molecules. 2018; 23(2):363. https://doi.org/10.3390/molecules23020363
Chicago/Turabian StyleYan, Qian-Qian, Shao-Jun Hu, Guang-Lu Zhang, Ting Zhang, Li-Peng Zhou, and Qing-Fu Sun. 2018. "Coordination-Enhanced Luminescence on Tetra-Phenylethylene-Based Supramolecular Assemblies" Molecules 23, no. 2: 363. https://doi.org/10.3390/molecules23020363
APA StyleYan, Q.-Q., Hu, S.-J., Zhang, G.-L., Zhang, T., Zhou, L.-P., & Sun, Q.-F. (2018). Coordination-Enhanced Luminescence on Tetra-Phenylethylene-Based Supramolecular Assemblies. Molecules, 23(2), 363. https://doi.org/10.3390/molecules23020363