New Terpenoids from Chamaecyparis formosensis (Cupressaceae) Leaves with Modulatory Activity on Matrix Metalloproteases 2 and 9
Abstract
:1. Introduction
2. Results and Discussion
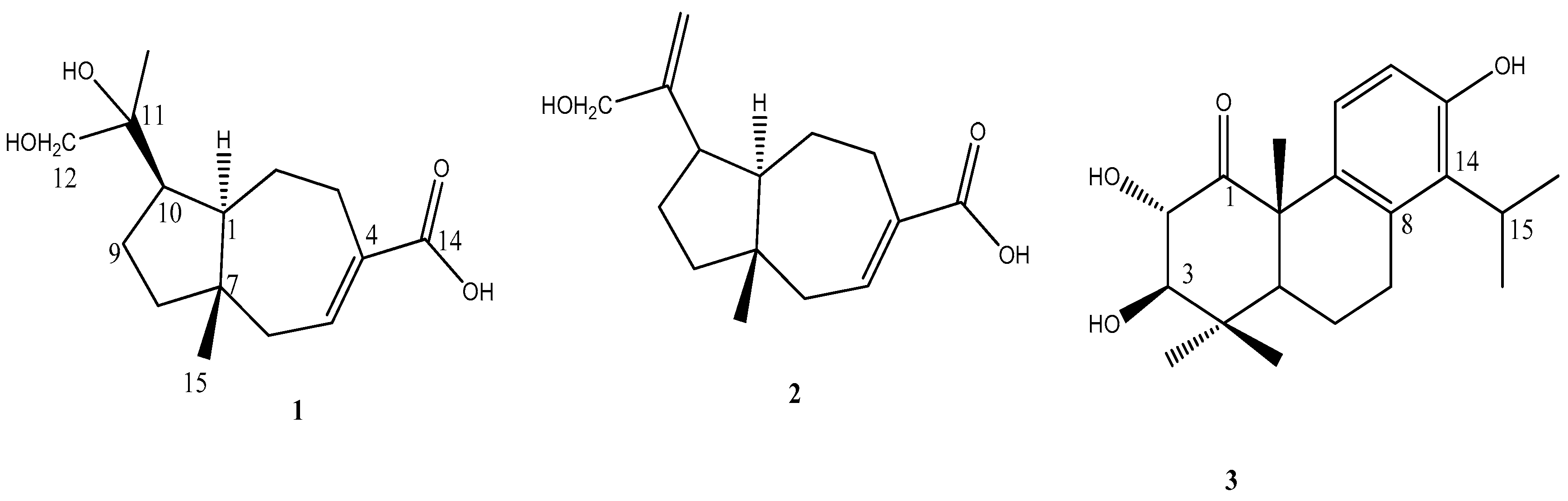
2.1. Compounds Isolated from the Leaves of C. formosensis
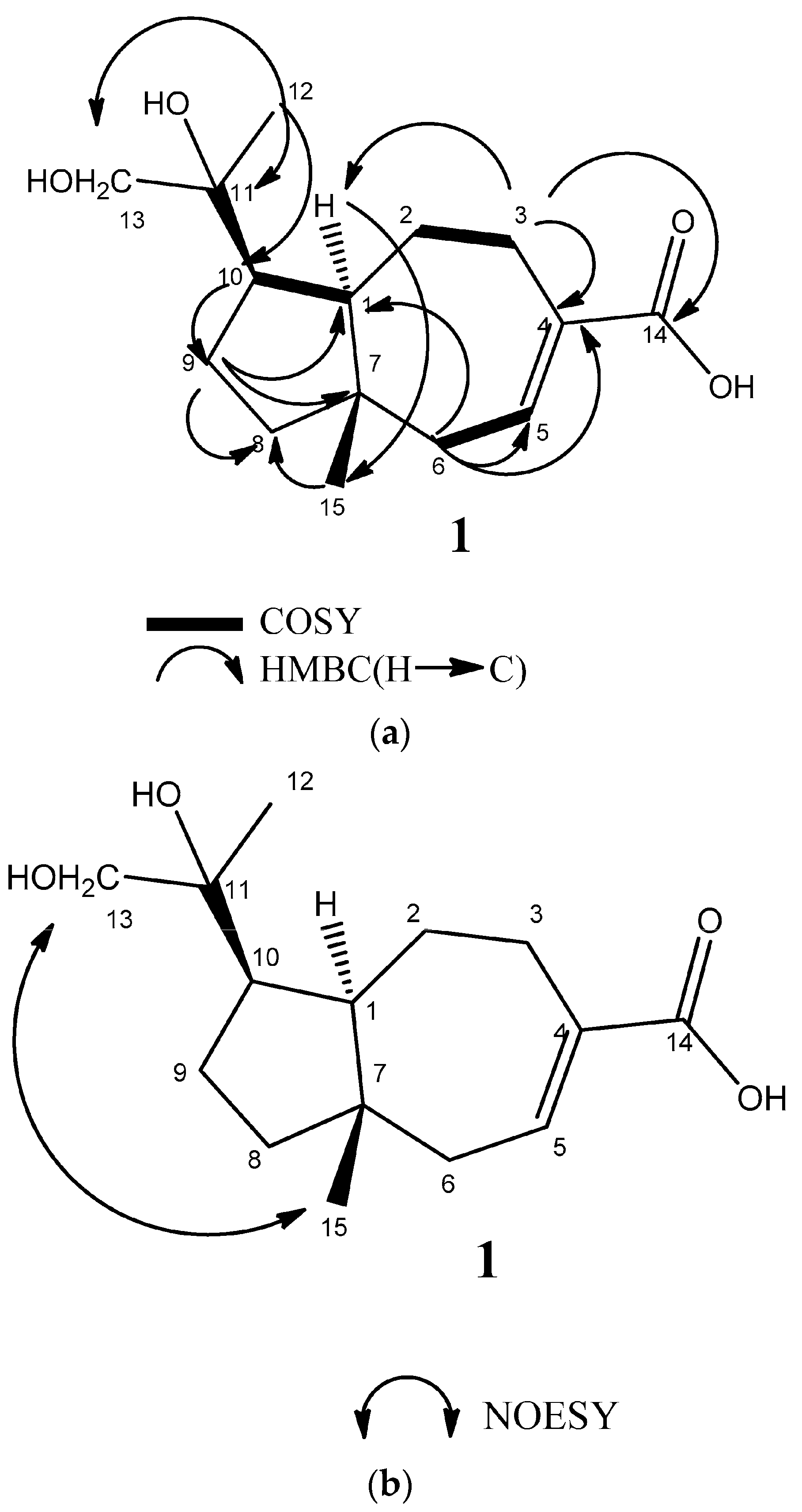
2.2. Structural Elucidation of Compounds 1–3
2.3. Evaluation of Modulatory Effects of Compounds on MMP-2 and MMP-9 Expression in HT-1080 Cells
3. Materials and Methods
3.1. General Methods
3.2. Plant Material
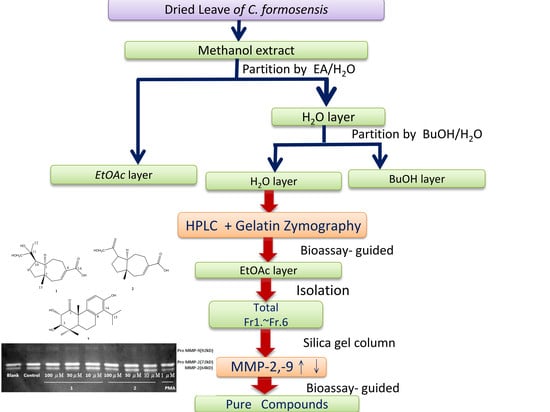
3.3. Extraction and Isolation
3.4. 11,12-Dihydroxyisodaucenoic Acid (1)
3.5. 12-Hydroxyisodaucenoic Acid (2)
3.6. 1-Oxo-2α,3β-dihydroxytotarol (3)
3.7. Cell Culture
3.8. Gelatin Zymography
3.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, W.P.; Hwang, C.Y.; Lin, T.P.; Hwang, S.Y. Historical biogeography and phylogenetic relationships of the genus Chamaecyparis (Cupressaceae) inferred from chloroplast DNA polymorphism. Plant Syst. Evol. 2003, 241, 13–28. [Google Scholar] [CrossRef]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Lee, S.G.; Choi, D.H.; Park, J.D.; Ahn, Y.J. Insecticidal activities of constituents identified in the essential oil from leaves of Chamaecyparis obtusa against Callosobruchus chinensis (L.) and Sitophilus oryzae (L.). J. Stored Prod. Res. 2003, 39, 375–384. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Kuo, Y.H.; Kuo, C.C.; Chen, L.T.; Cheung, C.H.; Chao, T.Y.; Lin, C.H.; Pan, W.Y.; Chang, C.Y.; Chien, S.C. Chamaecypanone C, a novel skeleton microtubule inhibitor, with anticancer activity by trigger caspase 8-Fas/FasL dependent apoptotic pathway in human cancer cells. Biochem. Pharmacol. 2010, 79, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lu, F.Y.; Ou, C.H. Trees of Taiwan. Monographic Publication No. 7; College of Agriculture, National Chung Hsing University: Taichung, Taiwan, 1988; pp. 91–92. [Google Scholar]
- Li, H.-L.; Keng, H. Flora of Taiwan, 2nd ed.; Huang, T.-C., Ed.; Epoch Publ. Co.: Taipei, Taiwan, 1994. [Google Scholar]
- Ho, C.L.; Hua, K.F.; Hsu, K.P.; Wang, E.I.C.; Su, Y.C. Composition and antipathogenic activities of the twig essential oil of Chamaecyparis formosensis from Taiwan. Nat. Prod. Commun. 2012, 7, 933–936. [Google Scholar] [PubMed]
- Wang, S.Y.; Wu, C.L.; Chu, F.H.; Chien, S.C.; Kuo, Y.H.; Shyur, L.F.; Chang, S.T. Chemical composition and antifungal activity of essential oil isolated from Chamaecyparis formosensis Matsum Wood. Holzforschung 2005, 59, 295–299. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lin, C.Y.; Chang, S.T. Antitermitic activities of wood essential oil and its constituents from Chamaecyparis formosensis. Wood Sci. Technol. 2016, 50, 663–676. [Google Scholar] [CrossRef]
- Kafuka, K.; Ichikawa, N. The volatile compounds from leaves of Chamaecyparis formosensis. Nippon Kagaku Kaishi 1931, 52, 222–228. [Google Scholar] [CrossRef]
- Nozoe, T.; Chen, Y.S.; Toda, T. The structure of chamaecynone—A novel norsesquitenoid from Chamaecyparis formosensis Matsum. Tetrahedron Lett. 1966, 31, 3663–3669. [Google Scholar] [CrossRef]
- Fang, J.M.; Lai, L.J.; Cheng, Y.S. The constituents of the leaves of Chamaecyparis formosensis. J. Chin. Chem. Soc. 1986, 33, 265–266. [Google Scholar] [CrossRef]
- Fang, J.M.; Sheu, C.M.; Cheng, Y.S. A study of the constituents of the bark of Chamaecyparis formosensis. J. Chin. Chem. Soc. 1986, 33, 245–249. [Google Scholar] [CrossRef]
- Hsu, K.C.; Fang, J.M.; Cheng, Y.S. Diterpenes from pericarps of Chamaecyparis formosensis. J. Nat. Prod. 1995, 58, 1592–1595. [Google Scholar] [CrossRef]
- Zitka, O.; Kukacka, J.; Krizkov, S.; Haska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.J.; Parks, W.C. Control of Matrix Metalloproteinase Catalytic Activity Matrix Biology. J. Int. Soc. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Gaggar, A.; Blalock, J.E. MMP generated matrikines. Matrix Biol. 2015, 44–46, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.R.; Mackay, A.R. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers 2014, 6, 240–296. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Manuel, J.A.; Gawronska-Kozak, B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol. 2006, 25, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C. Study on the Constituents of the Leaves of Chamecyparis formosensis Matsum. Ph.D. Thesis, Department of Chemistry, National Taiwan University, Taipei, Taiwan, 1997. [Google Scholar]
- Knapp, H.; Weigand, C.; Gloser, J.; Winterhalter, P. 2-Hydroxy-2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-en-8-one: Precursor of 8,9-Dehydrotheaspirone in White-Fleshed Nectarines. J. Agric. Food Chem. 1997, 45, 1309–1313. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topcu, G.; Chen, S.; Cai, P.; Snyder, J.K. A new abietane diterpene from Salvia wiedemannii Boiss. J. Org. Chem. 1991, 56, 7354–7356. [Google Scholar] [CrossRef]
- Sanchez, A.J.; Konopelski, J.P. Phenol benzylic epoxide to quinone methide electron reorganization: Synthesis of (f)-taxodone. J. Org. Chem. 1994, 59, 5445–5452. [Google Scholar] [CrossRef]
- Lin, T.C.; Fang, J.M.; Cheng, Y.S. Terpenes and lignans from leaves of Chamaecyparis formosensis. Phytochemistry 1999, 51, 793–801. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sanchez, J.F.; Alvarez-Manzaneda, E.J.; Mu˜noz, M.; Haidour, A. Diterpenoids and cyclolanostanolides from Abies marocana. Phytochemistry 1992, 31, 615–620. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nishimura, K.; Kurimoto, S.; Takaishi, Y. New 29-nor-cycloartanes with a 3,4-seco- and a novel 2,3-seco-structure from the leaves of Sinocalycanthus chinensis. Bioorg. Med. Chem. 2011, 19, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Braun, A.; Estévez-Reyes, R.; González-Pérez, J.A.; González, A.G. Busaliol and busalicifol, two new tetrahydrofuran liganans from Bupleurum Salicifolium. J. Nat. Prod. 1995, 58, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Nishibe, S.; Chiba, M.; Sakushima, A.; Hisada, S.; Yamanouchi, S.; Takido, M.; Sankawa, U.; Sakakibara, A. Introduction of an alcoholic hydroxyl group into 2,3-dibenzylbutyrolactone lignans with oxidizing agents and carbon-13 nuclear magnetic resonance spectra of the oxidation products. Chem. Pharm. Bull. 1980, 28, 850–860. [Google Scholar] [CrossRef]
- Sena Filho, J.G.; Nimmo, S.L.; Xavier, H.S.; Barbosa-Filho, J.M.; Cichewicz, R.H. Phenylethanoid and lignan glycosides from polar extracts of Lantana, a genus of verbenaceous plants widely used in traditional herbal therapies. J. Nat. Prod. 2009, 72, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Li, J.; Wang, N.L.; Yao, X.S. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.F.; Lee, S.S. Flavonoid composition in the leaves of Twelve Litsea and Neolitsea Plants. J. Chin. Chem. Soc. 2011, 58, 376–383. [Google Scholar] [CrossRef]
- Marcus, V.B.; dos Santos, J.B.; Juceni, P.D.; Jorge, M.D. Biflavonoids and other phenolics from Caesalpinia pyramidalis (Fabaceae). J. Braz. Chem. Soc. 2005, 16, 1402–1405. [Google Scholar]
- Velandia, J.R.; de Carvalho, M.G.; Braz-Filho, R.; Werle, A.A. Biflavonoids and a Glucopyranoside Derivative from Ouratea semiserrata. Phytochem. Anal. 2002, 13, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.S.; Mitra, C.R. Putraflavone, a new biflavonoid from Putranjiva roxburghii. Phytochemistry 1971, 10, 2787–2791. [Google Scholar] [CrossRef]
- Li, S.H.; Zhang, H.J.; Niu, X.M.; Yao, P.; Sun, H.D.; Fong, H.S. Chemical Constituents from Amentotaxus yunnanensis and Torreya yunnanensis. J. Nat. Prod. 2003, 66, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Harborne, J.B.; Tomas-Barberan, F.A. Biflavonoids in the primitive monocots Isophysis tasmanica and Xerophyta plicata. Phyrochemistry 1987, 26, 2553–2555. [Google Scholar] [CrossRef]
- Pakrashi, A.; Kabir, S.N.; Ray, H. 3-(4-Hydroxyphenyl)-2-propenoicacid-A reproductive inhibitor in male rat. Contraception 1981, 23, 677–686. [Google Scholar] [CrossRef]
- Matsumoto, K.; Feng, C.; Handa, S.; Oguma, T.; Katsuki, T. Asymmetric epoxidation of (Z)-enol esters catalyzed by titanium(salalen) complex with aqueous hydrogen peroxide. Tetrahedron 2011, 67, 6474–6478. [Google Scholar] [CrossRef]
- Zeng, B.B.; Wu, Y.; Jiang, S.; Yu, Q.; Yao, Z.J.; Liu, Z.H.; Li, H.Y.; Li, Y.; Chen, X.G.; Wu, Y.L. Studies on mimicry of naturally occurring annonaceous acetogenins: Non-THF analogues leading to remarkable selective cytotoxicity against human tumor cells. Chem. Eur. J. 2003, 9, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Jiang, M.; Wang, J.; Fu, H. General copper-catalyzed transformations of functional groups from arylboronic acids in water. Chem. Eur. J. 2011, 17, 5652–5660. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.A.; Ahmad, A.S.; Nafady, A.M.; Mansour, A.I. Chemical composition of the stem bark and leaves of Ficus pandurata Hance. Nat. Prod. Res. 2009, 23, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Hashidoko, Y.; Tashara, S.; Mizutani, J. Carotanoids and an Acoranoid from Rosa rugosa Leaves. Phytochemistry 1991, 30, 3729–3739. [Google Scholar] [CrossRef]
- Ma, W.H.; Huang, H.; Zhou, P.; Chen, D.F. Schisanwilsonenes A-C, anti-HBV carotane sesquiterpenoids from the fruits of Schisandra wilsoniana. J. Nat. Prod. 2009, 72, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Lin, J.K. EGCG inhibits the invavion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/Marrest. J. Agric. Food Chem. 2011, 59, 13318–13327. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Chen, P.N.; Kuo, W.H.; Wang, J.W.; Chu, S.C.; Hsieh, Y.S. Antimetastatic potentials of phyllanthusurinaria L on A549 and Lewis lung carcinoma cells via repression of matrix-degrading proteases. Integr. Cancer Ther. 2011, 10, 341–349. [Google Scholar]
- Lee, C.W.; Choi, H.J.; Kim, H.S.; Kim, D.H.; Chang, I.S.; Moon, H.T.; Lee, S.Y.; Oh, W.K.; Woo, E.R. Biflavonoids isolated from Selaginella tamariscina regulate the expression of matrix metalloproteinase in human skin fibroblasrs. Bioorg. Med. Chem. 2008, 16, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Miksztowicz, V.; Schreier, L. Metalloproteinases in metabolic syndrome. Clin. Chim. Acta 2011, 412, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 1.99 (1H, m) | 58.0 | 1.89 (1H, m) | 57.2 |
| 2 | 1.48 (1H, m) 2.54 (1H, m) | 24.2 | 1.25 (1H, m) 1.54 (1H, m) | 24.0 |
| 3 | 1.80 (1H, m) 3.08 (1H, m) | 30.9 | 3.03 (1H, m) 1.90 (1H, m) | 28.4 |
| 4 | 136.4 | 134.5 | ||
| 5 | 7.10 (1H, m) | 142.2 | 7.20 (1H, m) | 144.8 |
| 6 | 2.40 (1H, m) 2.10 (1H, m) | 43.3 | 2.45 (1H, dd, J = 14.0, 9.6) 2.03 (1H, m) | 43.0 |
| 7 | 42.7 | 42.9 | ||
| 8 | 1.38 (1H, m) 1.48 (1H, m) | 42.4 | 1.58 (1H, br dd, J = 11.6, 6.7) 1.46 (1H, br dd, J = 11.6, 6.7) | 42.5 |
| 9 | 1.65 (1H, m) 1.78 (1H, m) | 27.7 | 1.70 (2H, m) | 30.1 |
| 10 | 2.59 (1H, m) | 48.6 | 2.91 (1H, m) | 45.6 |
| 11 | 75.9 | 151.7 | ||
| 12 | 3.28 (1H, d, J = 10.4 Hz) 3.33 (1H, d, J = 10.4 Hz) | 70.9 | 4.06 (1H, d, J = 14) 3.98 (1H, d, J = 14) | 67.7 |
| 13 | 1.10 (3H, s) | 22.0 | 4.93 (1H, br s), 5.16 (1H, br s) | 111.4 |
| 14 | 169.6 | 172.8 | ||
| 15 | 0.80 (3H, s) | 19.7 | 0.81 (3H, s, CH3) | 19.9 |
| Compound | MMP-2 | MMP-9 |
|---|---|---|
| PMA | 2.5 * (Pro MMP-9 **, 1 μM ***) | |
| EGCG | 0.2–0.4 (Pro MMP-2, 100 μM) | |
| 13-Hydroxyisodaucenoic acid (2) | 1.26 (50 µM) 1.32 (10 µM) | |
| Nortrachelogenin (18) | 1.30 (50 µM) 1.59 (10 µM) | 1.54 (10 µM) |
| 8′β-hydroxynortrachelogenin (19) | 1.23 (50 µM) 1.34 (10 µM) | |
| Epicatechin (23) | 1.31 (50 µM) 1.40 (10 µM) | |
| Catechin (24) | 1.29 (50 µM) 1.17 (10 µM) | |
| 4′′′-O-methylametoflavone (26) | 1.42 (Pro MMP-9 ***, 10 µM) | |
| 4′′′,7-dimethoxyametoflavone (27) | 1.18 (Pro-MMP-9, 50 µM) |
| Position | δH (mult., J in Hz) | δC |
|---|---|---|
| 1 | 210.4 | |
| 2 | 4.70 (1H, dd, J = 10.4, 5.5 Hz) | 75.4 |
| 3 | 3.05 (1H, d, J = 10.4 Hz) | 85.5 |
| 4 | 38.6 | |
| 5 | 1.45 (1H, dd, J = 12.1, 1.2 Hz) | 49.8 |
| 6 | 1.55 (1H, m) | 18.7 |
| 2.02 (1H, m) | ||
| 7 | 2.67 (1H, m) | 29.9 |
| 2.95 (1H, m) | ||
| 8 | 132.5 | |
| 9 | 131.3 | |
| 10 | 53.5 | |
| 11 | 6.98 (1H, d, J = 8.5 Hz) | 128.9 |
| 12 | 6.57 (1H, d, J = 8.5 Hz) | 114.4 |
| 13 | 153.1 | |
| 14 | 132.5 | |
| 15 | 3.20 (1H, sept) | 29.6 |
| 16 | 1.30 (3H, d, J = 6.7 Hz) | 20.5 |
| 17 | 1.31 (3H, d, J = 6.7 Hz) | 20.4 |
| 18 | 1.14 (3H, s) | 29.0 |
| 19 | 1.15 (3H, s) | 17.0 |
| 20 | 1.61 (3H, s) | 25.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-L.; Mei, H.-C.; Kuo, I.-C.; Hsiao, G.; Kuo, Y.-H.; Lee, C.-K. New Terpenoids from Chamaecyparis formosensis (Cupressaceae) Leaves with Modulatory Activity on Matrix Metalloproteases 2 and 9. Molecules 2018, 23, 604. https://doi.org/10.3390/molecules23030604
Chang M-L, Mei H-C, Kuo I-C, Hsiao G, Kuo Y-H, Lee C-K. New Terpenoids from Chamaecyparis formosensis (Cupressaceae) Leaves with Modulatory Activity on Matrix Metalloproteases 2 and 9. Molecules. 2018; 23(3):604. https://doi.org/10.3390/molecules23030604
Chicago/Turabian StyleChang, Meng-Lun, Hui-Ching Mei, I-Chih Kuo, George Hsiao, Yueh-Hsiung Kuo, and Ching-Kuo Lee. 2018. "New Terpenoids from Chamaecyparis formosensis (Cupressaceae) Leaves with Modulatory Activity on Matrix Metalloproteases 2 and 9" Molecules 23, no. 3: 604. https://doi.org/10.3390/molecules23030604