From the Synthesis of Nanovehicles to Participation in the First Nanocar Race—View from the French Team
Abstract
:1. Introduction
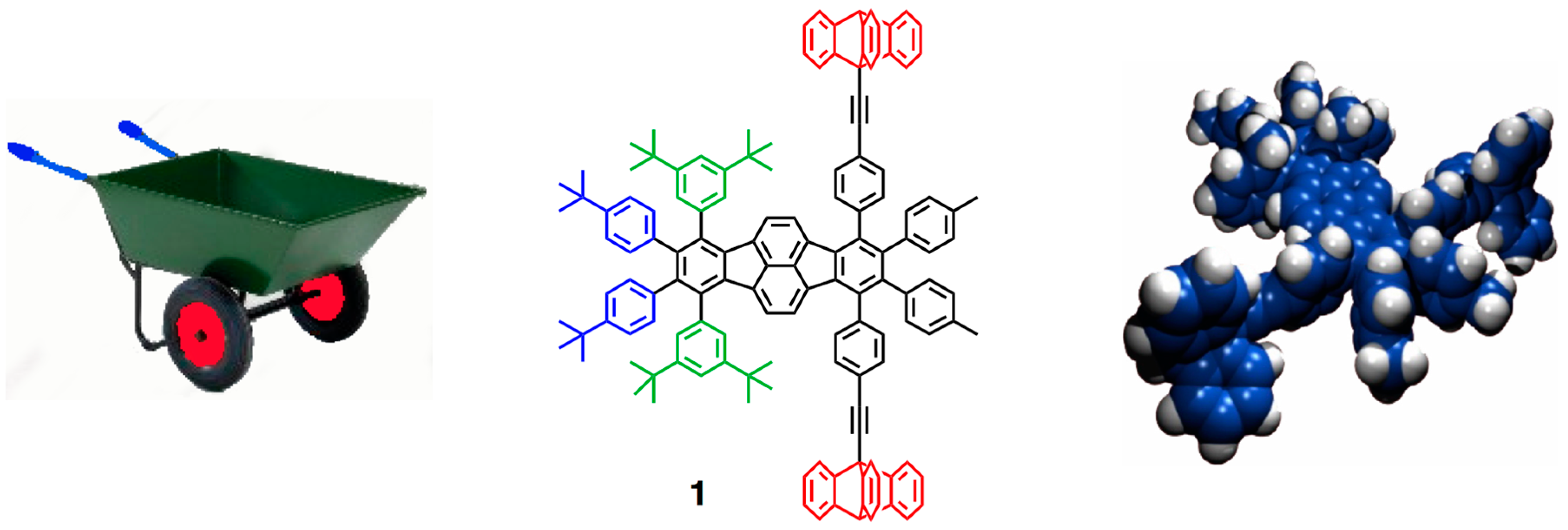
2. The First Two-Wheeled and Two-Legged Vehicle: The Molecular Wheelbarrow
2.1. Design
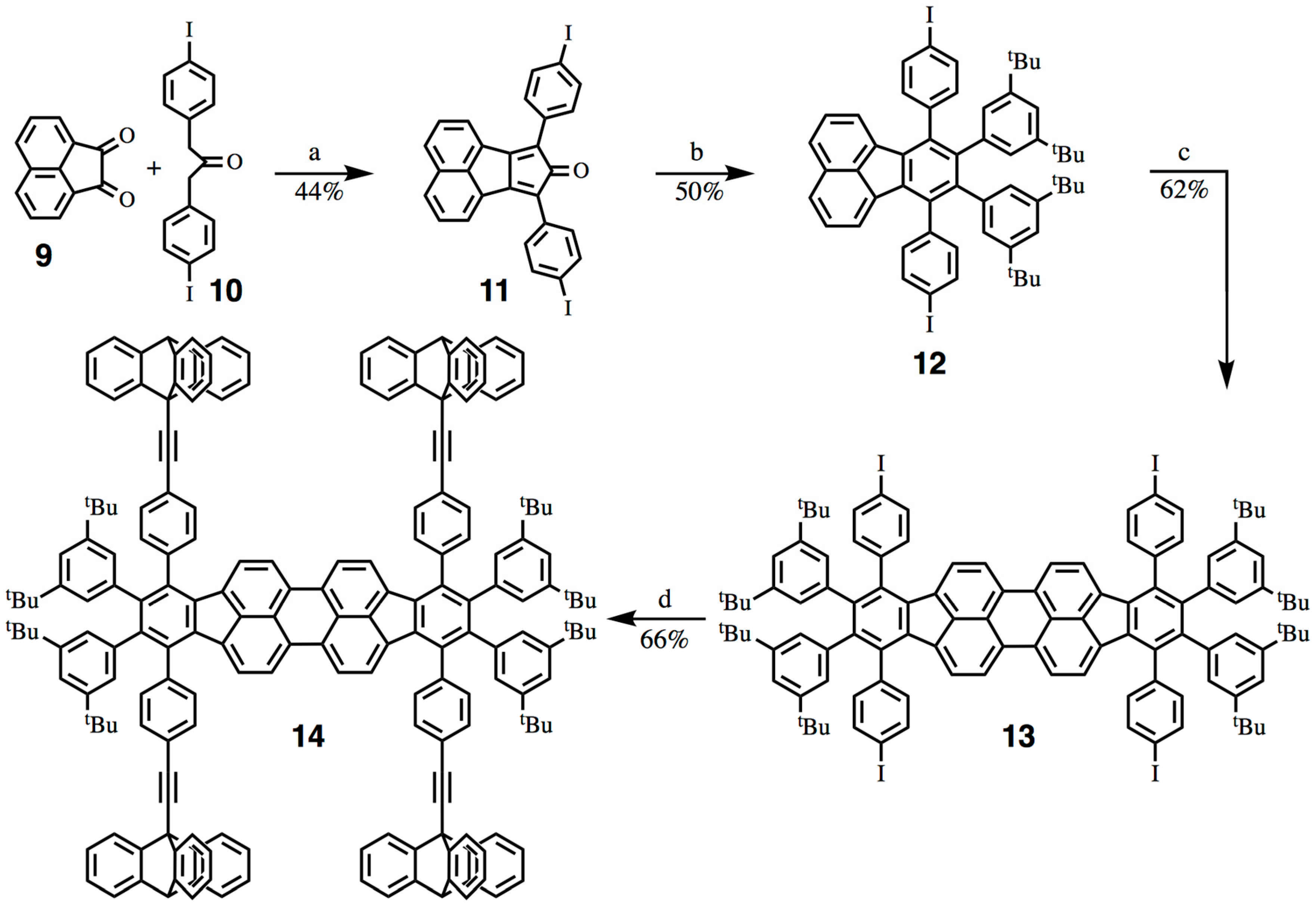
2.2. Synthesis
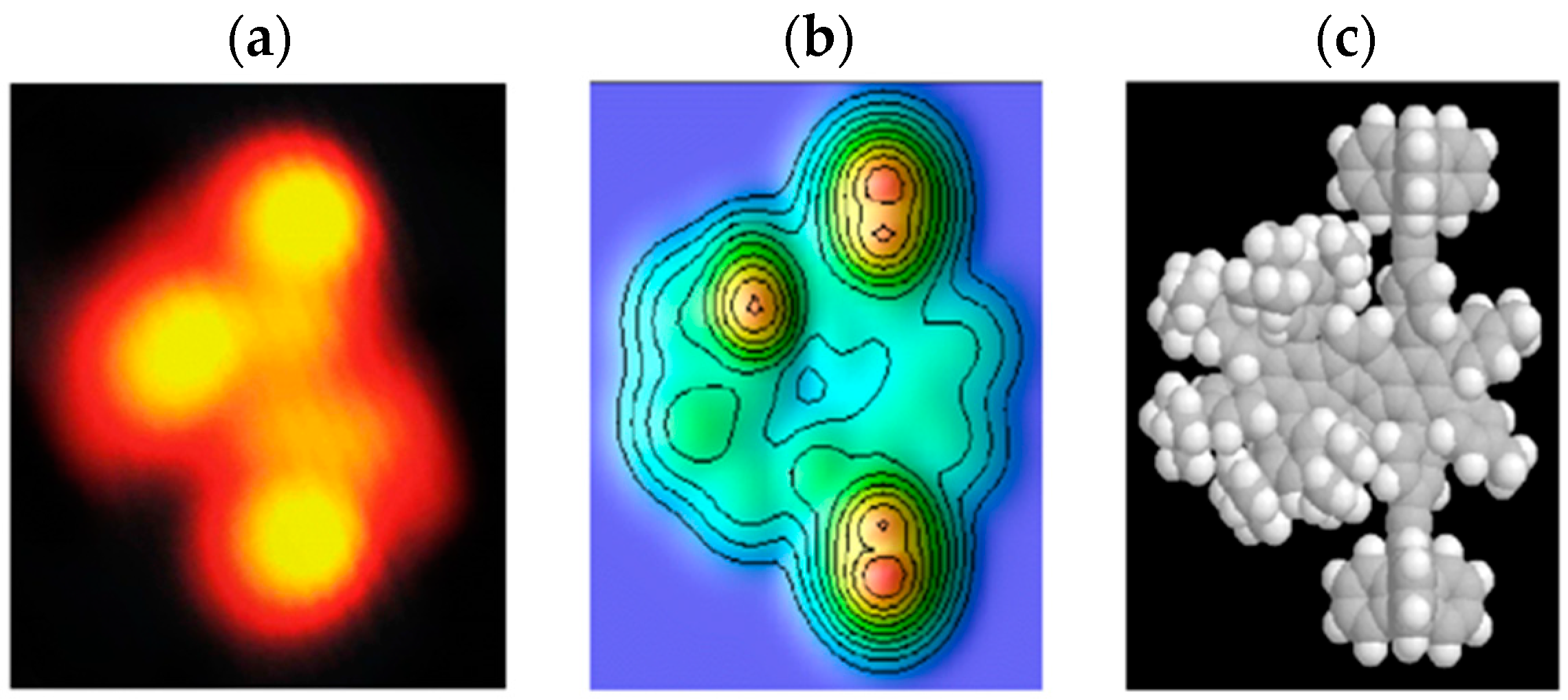
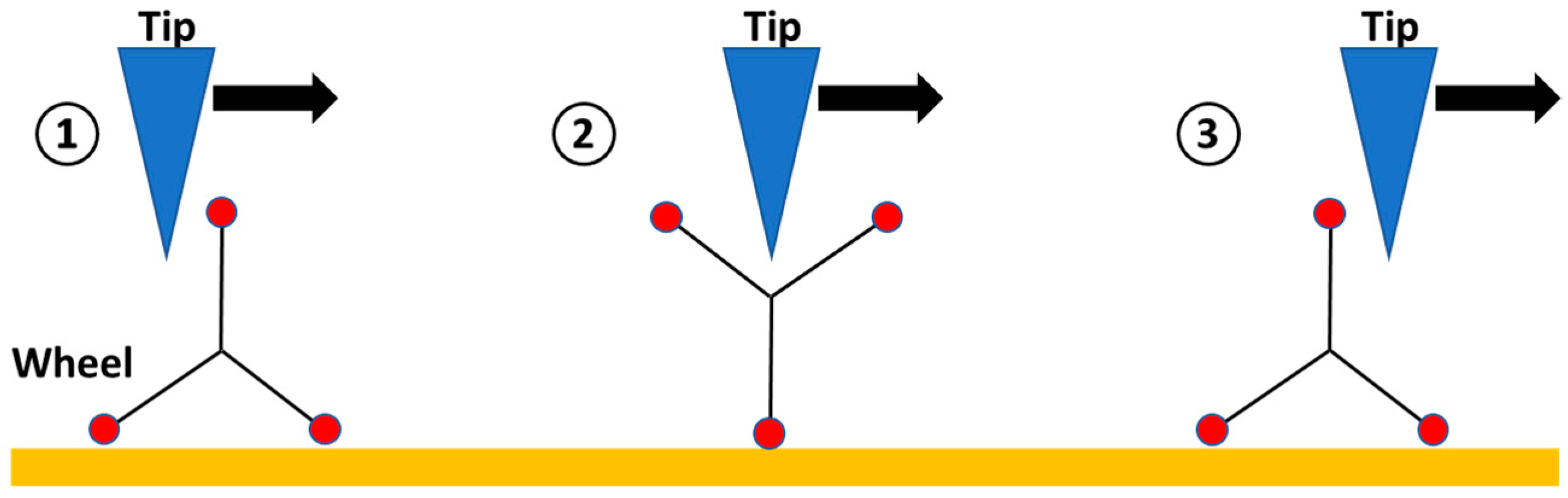
2.3. Single Molecule Scanning Tunneling Microscopy Images and Manipulation
3. The First Rotation of Wheels on a Surface
3.1. Synthesis and Deposition of a Bis(ethynyltriptycene) as Prototype of a Wheel Dimer
3.2. Unidirectional Rotation of the Wheel
4. A Planar Nanocar
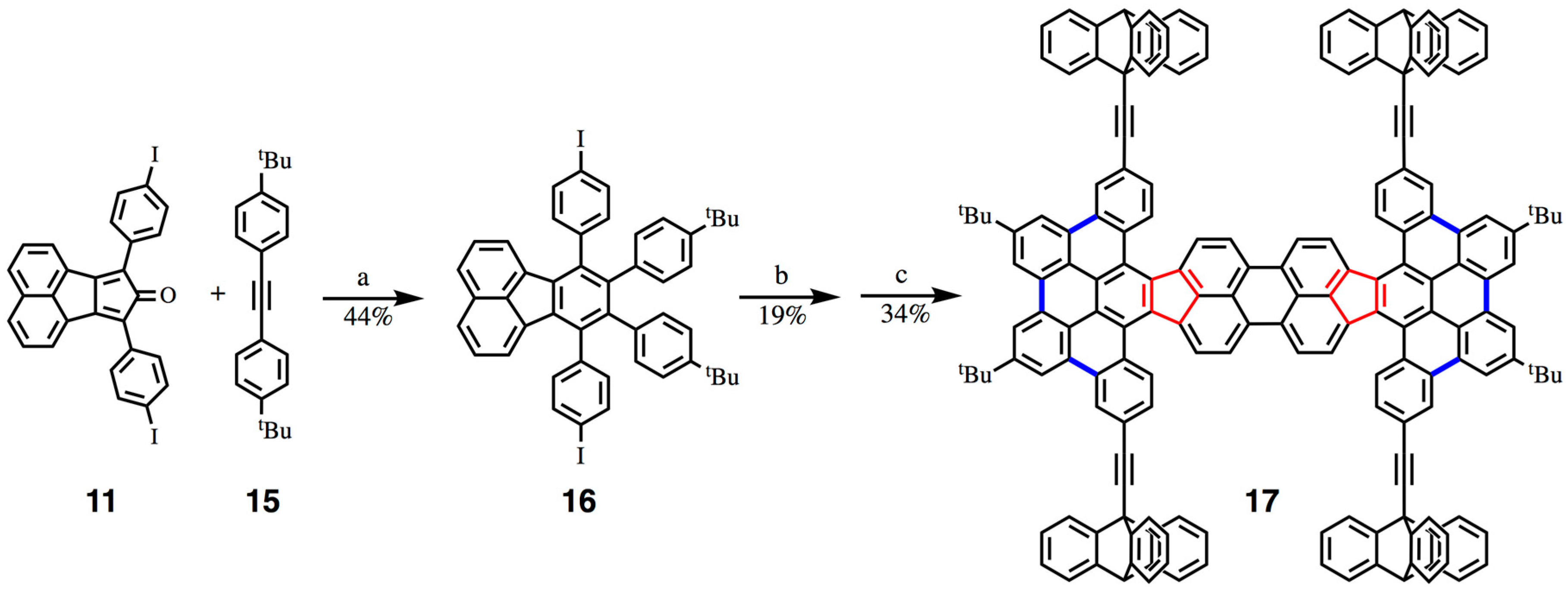
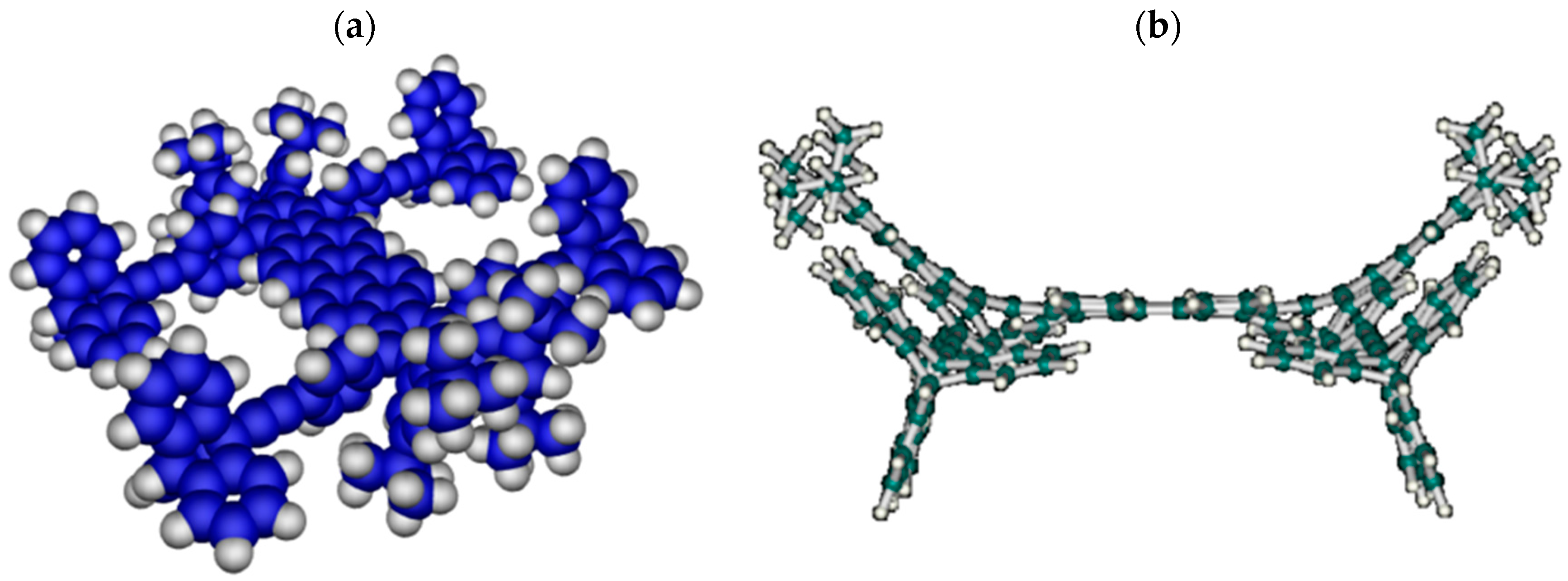
5. A Curved Nanocar
6. Participation in the First Nanocar Race: The View from the French Team
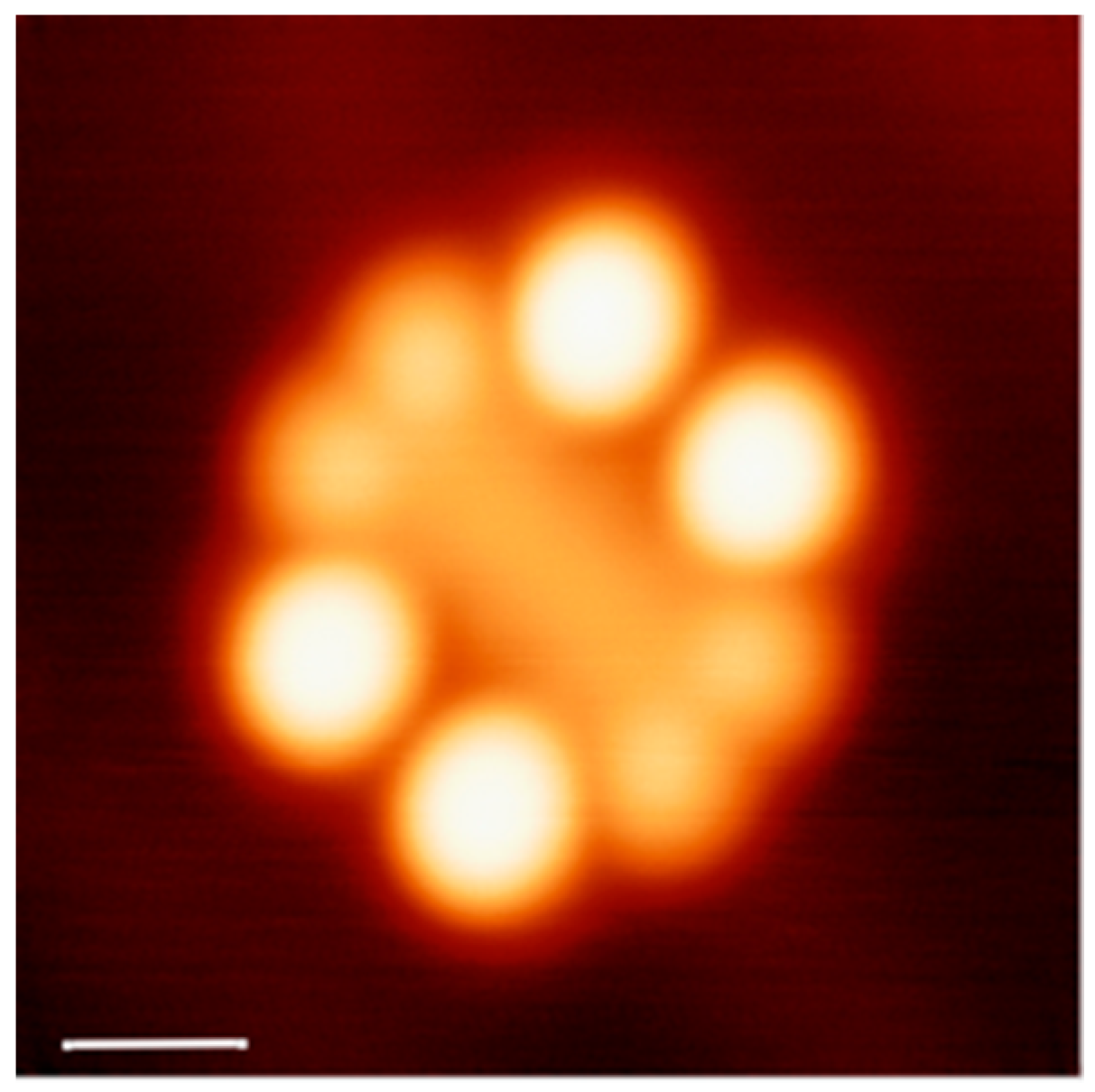
6.1. Deposition and Imaging of the French Nanocar
6.2. The Race and the Official Ranking
7. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References and Notes
- Joachim, C.; Rapenne, G. Molecule concept nanocars: Chassis, wheels and motors? ACS Nano 2013, 7, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Nanocar Race Official Website. Available online: http://nanocar-race.cnrs.fr/indexEnglish.php (accessed on 7 January 2018).
- Research of the Year 2017. Chemical Engineering News. 18 December 2017. Available online: https://cen.acs.org/articles/95/i49/chemistry-research-of-the-year-2017.html (accessed on 7 January 2018).
- 2017, Une Année de Sciences en Images. Le Monde. Available online: http://www.lemonde.fr/sciences/portfolio/2017/12/26/2017-une-annee-de-sciences-en-images_5234475_1650684.html (accessed on 7 January 2018).
- Rapenne, G. Synthesis of technomimetic molecules: Towards rotation control in single molecular machines and motors. Org. Biomol. Chem. 2005, 3, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Ma, Y.X. Iptycenes Chemistry from Synthesis to Applications; Springer-Verlag: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Joachim, C.; Moresco, F.; Rapenne, G.; Meyer, G. The design of a nanoscale molecular barrow. Nanotechnology 2002, 13, 330–335. [Google Scholar] [CrossRef]
- Jimenez-Bueno, G.; Rapenne, G. Technomimetic molecules: Synthesis of a molecular wheelbarrow. Tetrahedron Lett. 2003, 44, 6261–6263. [Google Scholar] [CrossRef]
- Shirai, Y.; Osgood, A.J.; Zhao, Y.; Kelly, K.F.; Tour, J.M. Directional control in thermally driven single-molecule nanocars. Nano Lett. 2005, 5, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.-F.; Sasaki, T.; Shirai, Y.; Guerrero, J.M.; Tour, J.M. Synthetic Routes toward Carborane-Wheeled Nanocars. J. Org. Chem. 2007, 72, 9481–9490. [Google Scholar] [CrossRef] [PubMed]
- Vives, G.; Tour, J.M. Synthesis of a Nanocar with Organometallic Wheels. Tetrahedron Lett. 2009, 50, 1427–1430. [Google Scholar] [CrossRef]
- Chu, P.L.E.; Wang, L.Y.; Khatua, S.; Kolomeisky, A.B.; Link, S.; Tour, J.M. Synthesis and Single-Molecule Imaging of Highly Mobile Adamantane-Wheeled Nanocars. ACS Nano 2013, 7, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jacquot de Rouville, H.P.; Garbage, R.; Ample, F.; Nickel, A.; Meyer, J.; Moresco, F.; Joachim, C.; Rapenne, G. Synthesis and STM imaging of symmetric and dissymmetric ethynyl-bridged dimers of boron-subphthalocyanine bowl-shaped nano-wheels. Chem. Eur. J. 2012, 18, 8925–8928. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Meyer, J.; Ohmann, R.; Jacquot de Rouville, H.-P.; Rapenne, G.; Joachim, C.; Cuniberti, G.; Moresco, F. STM manipulation of boron-subphthalocyanine nano-wheel dimers on Au(111). J. Phys. Condens. Matter 2012, 24, 404001. [Google Scholar] [CrossRef] [PubMed]
- Kudernac, T.; Ruangsupapichat, N.; Parschau, M.; Maci, B.; Katsonis, N.; Harutyunyan, S.R.; Ernst, K.H.; Feringa, B.L. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature 2011, 479, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Kotturi, K.; Raeisi, M.; Latt, K.Z.; Zhang, Y.; Perumal, K.; Rabbani, R.; Sarkar, S.; Li, Y.; Hla, S.-W.; Masson, E. Anatomy of a Supramolecular Nanowagon. In preparation.
- Soe, W.H.; Shirai, Y.; Durand, C.; Yonamine, Y.; Minami, K.; Bouju, X.; Kolmer, M.; Ariga, K.; Joachim, C.; Nakanishi, W. Conformation Manipulation and Motion of a Double Paddle Molecule on an Au(111) Surface. ACS Nano 2017, 11, 10357–10365. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.; Moresco, F.; Launay, J.P.; Joachim, C. Training for the 1st international nano-car race: The Dresden molecule-vehicle. Eur. Phys. J. Appl. Phys. 2016, 76, 10001. [Google Scholar] [CrossRef]
- Pawlak, R.; Meier, T.; Renaud, N.; Kisiel, M.; Hinaut, A.; Glatzel, T.; Sordes, D.; Durand, C.; Soe, W.H.; Baratoff, A.; et al. Design and Characterization of an Electrically Powered Single Molecule on Gold. ACS Nano 2017, 11, 9930–9940. [Google Scholar] [CrossRef] [PubMed]
- Toyota, S. Rotational isomerism involving acetylene carbon. Chem. Rev. 2010, 110, 5398–5424. [Google Scholar] [CrossRef] [PubMed]
- Rapenne, G.; Jimenez-Bueno, G. Molecular machines: Synthesis and characterization of two prototypes of molecular wheelbarrows. Tetrahedron 2007, 63, 7018–7026. [Google Scholar] [CrossRef]
- Rapenne, G.; Grill, L.; Zambelli, T.; Stojkovic, S.; Ample, F.; Moresco, F.; Joachim, C. Launching and landing single molecular wheelbarrows on a Cu (100) surface. Chem. Phys. Lett. 2006, 431, 219–222. [Google Scholar] [CrossRef]
- Grill, L.; Rieder, K.H.; Moresco, F.; Jimenez-Bueno, G.; Wang, C.; Rapenne, G.; Joachim, C. Imaging of a molecular wheelbarrow by scanning tunneling microscopy. Surf. Sci. 2005, 584, 153–158. [Google Scholar] [CrossRef]
- For early examples of mechanically-induced motions of molecules, see: Moresco, F.; Meyer, G.; Rieder, K.-H.; Tang, H.; Gourdon, A.; Joachim, A. Low temperature manipulation of big molecules in constant height mode. Appl. Phys. Lett. 2001, 78, 306–308. [Google Scholar], doi:10.1063/1.1339251 and the references cited.
- Grill, L.; Rieder, K.H.; Moresco, F.; Rapenne, G.; Stojkovic, S.; Bouju, X.; Joachim, C. Rolling a single molecular wheel at the atomic scale. Nat. Nanotechnol. 2007, 2, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Jacquot de Rouville, H.P.; Garbage, R.; Cook, R.E.; Pujol, A.R.; Sirven, A.M.; Rapenne, G. Synthesis of polycyclic aromatic hydrocarbon-based nanovehicles equipped with triptycene wheels. Chem. Eur. J. 2012, 18, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Rapenne, G.; Joachim, C. The first nanocar race. Nat. Rev. Mater. 2017, 2, 17040. [Google Scholar] [CrossRef]
- The rules of the Nanocar Race have been published in: Joachim, C.; Durand, C.; Launay, J.-P.; Rapenne, G.; Kammerer, C.; Jacquot de Rouville, H.-P.; Garbage, R.; Martrou, D.; Soe, W.-H.; Gauthier, S.; et al. The NanoCar Race: The First International Molecule-Cars Race. L’actualité Chimique. October 2016, n°411, Page V. It is mentioned that the race will take place on a small portion of Au (111) and that the nanocar must present a chemical structure of minimum 100 atoms.
- Pawlak, R.; Meier, T. Fast and curious. Nat. Nanotechnol. 2017, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.J.; García-López, V.; Petermeier, P.; Grill, L.; Tour, J.M. How to Build and Race a Fast Nanocar. Nat. Nanotechnol. 2017, 12, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Makoudi, Y.; Duverger, E.; Arab, M.; Chérioux, F.; Ample, F.; Rapenne, G.; Bouju, X.; Palmino, F. Room temperature electronic template effect of the Sm/Si(111)-8x2 interface for self-alignment of organic molecules. Chem. Phys. Chem. 2008, 9, 1437–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouju, X.; Chérioux, F.; Coget, S.; Rapenne, G.; Palmino, F. Directional molecular sliding at room temperature on a silicon runway. Nanoscale 2013, 5, 7005–7010. [Google Scholar] [CrossRef] [PubMed]
- Chérioux, F.; Palmino, F.; Galangau, O.; Rapenne, G. Controlled directional motions of molecular vehicles, rotors and motors: From metallic to silicon surfaces, a strategy to operate at higher temperature. Chem. Phys. Chem. 2016, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- “Course des Garçons de Café” (Waiter’s Race) Wikipedia Webpage. Available online: https://en.wikipedia.org/wiki/Waiters%27_Race (accessed on 7 January 2018).


| MANA NIMS | Nano Dragster | Nano-Wagon | Dipolar Racer | Nano-Windmill | Green Buggy | |
|---|---|---|---|---|---|---|
| Country | Japan | Switzerland | USA | Austria & USA | Germany | France |
| Team leader | Waka Nakanichi | Rémy Pawlack | Saw Wai Hla & Eric Masson | Leonhard Grill & James Tour | Francesca Moresco | Gwénaël Rapenne |
| Surface | Au(111) | Au(111) | Au(111) | Ag(111) | Au(111) | Au(111) |
| Number of atoms | 88 atoms (C50H34O4) | 42 atoms (C22H17N3) | 642 atoms (C244H222N120056) | 72 atoms (C32H36N2O2) | 108 atoms (C56H48O4) | 300 atoms (C184H116) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacquot de Rouville, H.-P.; Kammerer, C.; Rapenne, G. From the Synthesis of Nanovehicles to Participation in the First Nanocar Race—View from the French Team. Molecules 2018, 23, 612. https://doi.org/10.3390/molecules23030612
Jacquot de Rouville H-P, Kammerer C, Rapenne G. From the Synthesis of Nanovehicles to Participation in the First Nanocar Race—View from the French Team. Molecules. 2018; 23(3):612. https://doi.org/10.3390/molecules23030612
Chicago/Turabian StyleJacquot de Rouville, Henri-Pierre, Claire Kammerer, and Gwénaël Rapenne. 2018. "From the Synthesis of Nanovehicles to Participation in the First Nanocar Race—View from the French Team" Molecules 23, no. 3: 612. https://doi.org/10.3390/molecules23030612












