Efficient Separation of Four Antibacterial Diterpenes from the Roots of Salvia Prattii Using Non-Aqueous Hydrophilic Solid-Phase Extraction Followed by Preparative High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus
2.2. Reagents and Stationary Phases
2.3. Preparation of the Crude Sample
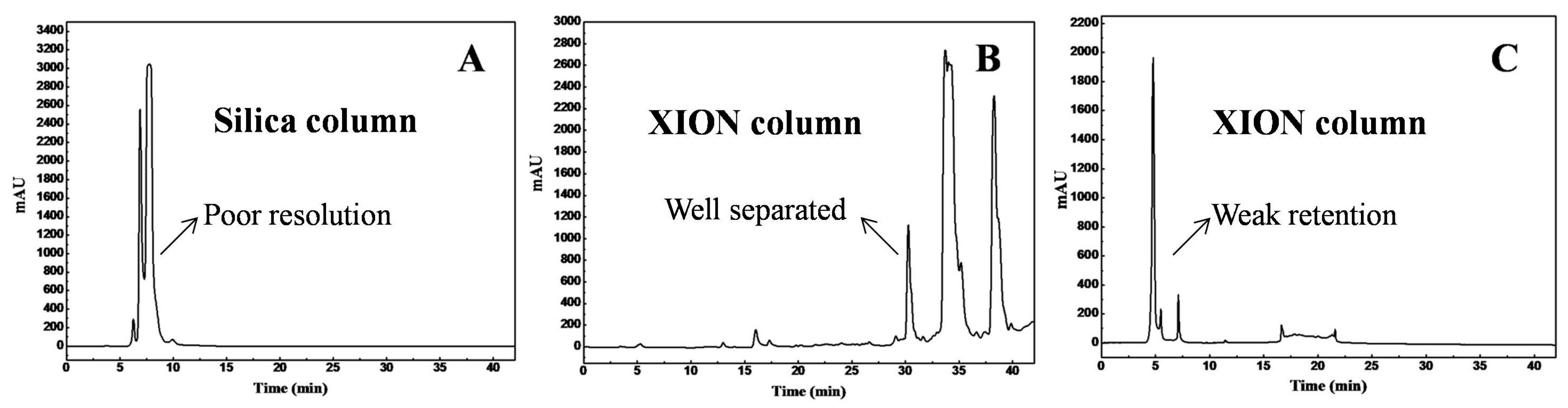
2.4. HILIC-SPE Pre-Separation
2.5. Antibacterial Activity
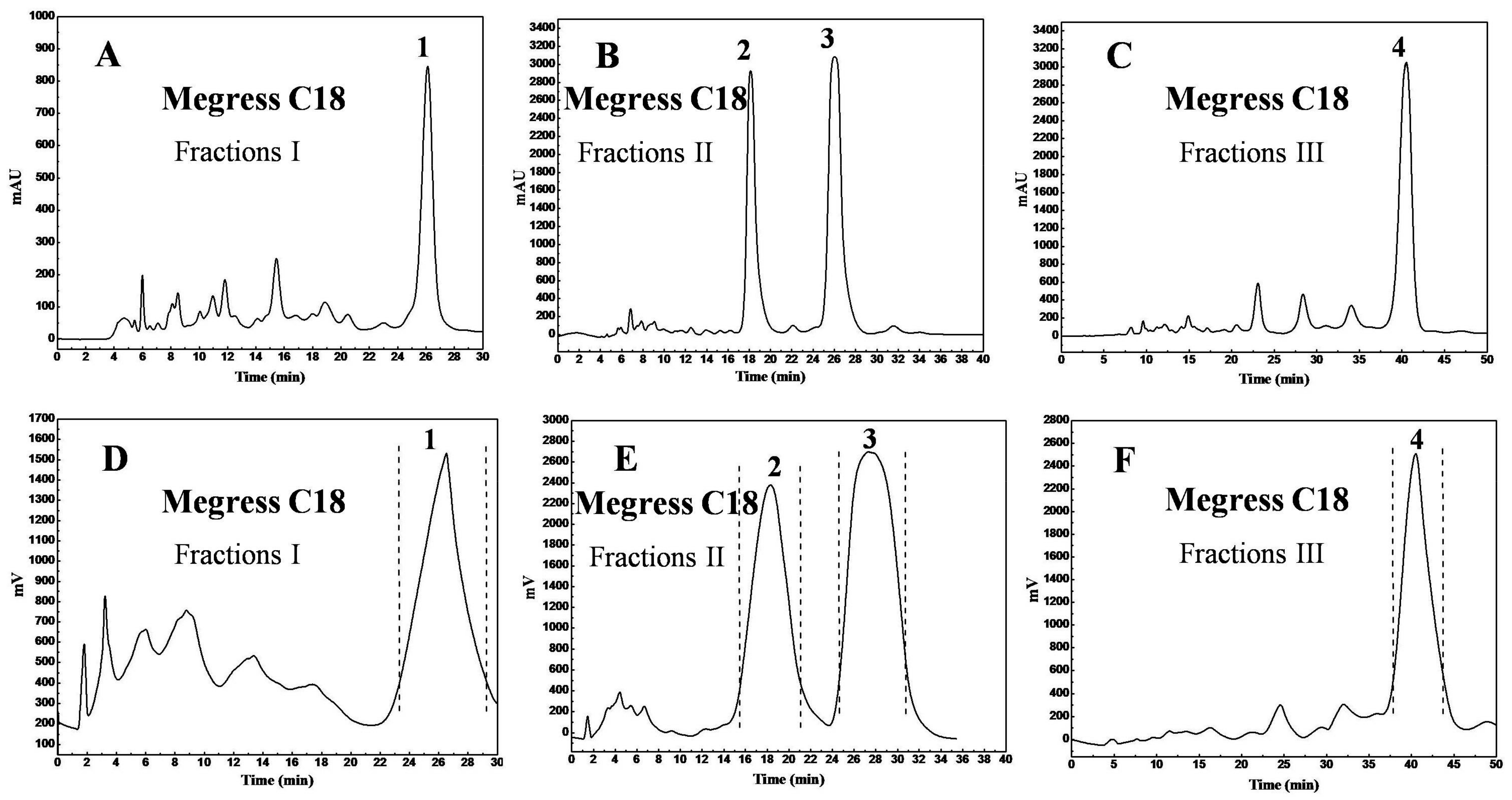
2.6. Purification of the Main Diterpenes by Prep-HPLC
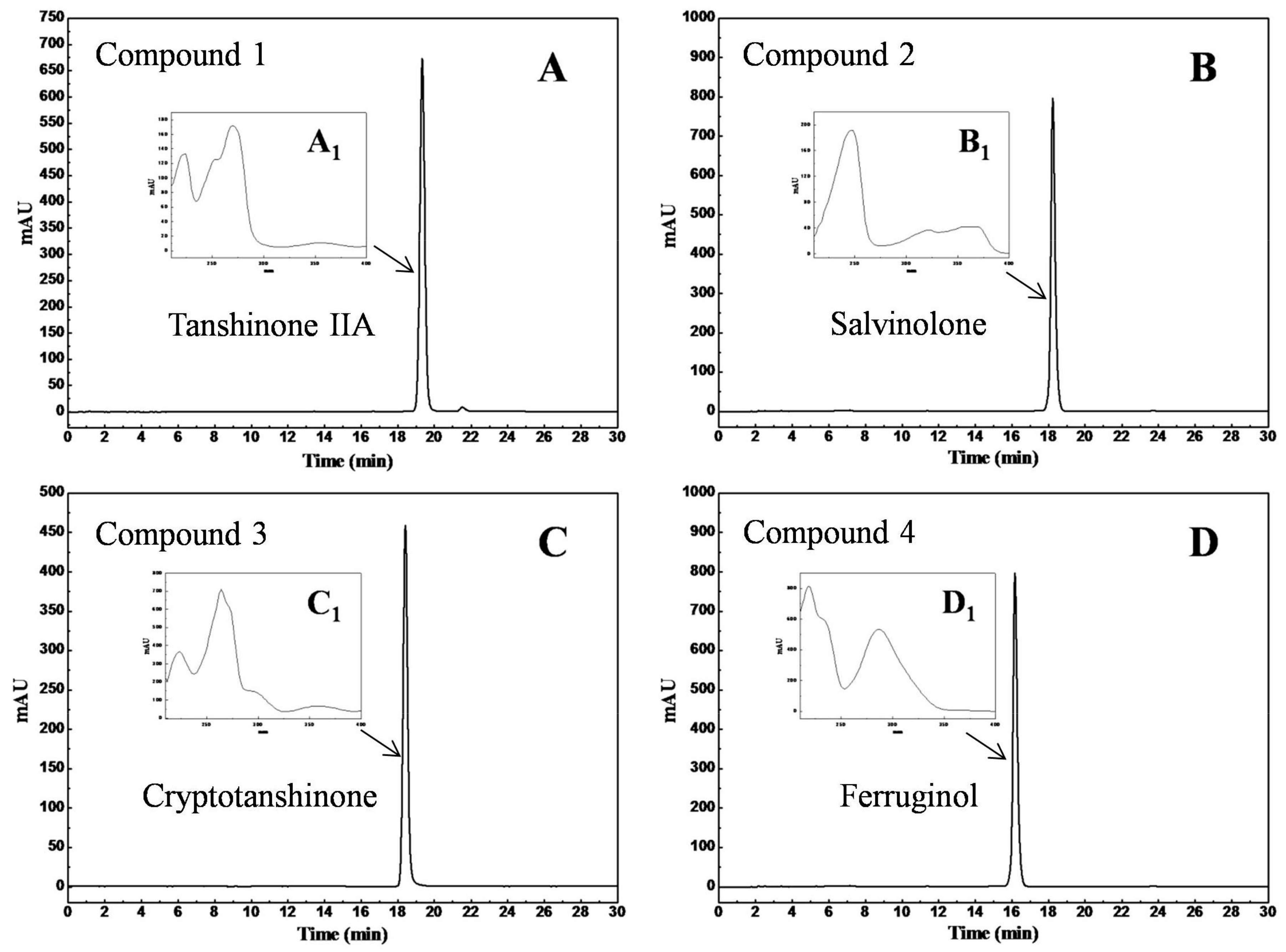
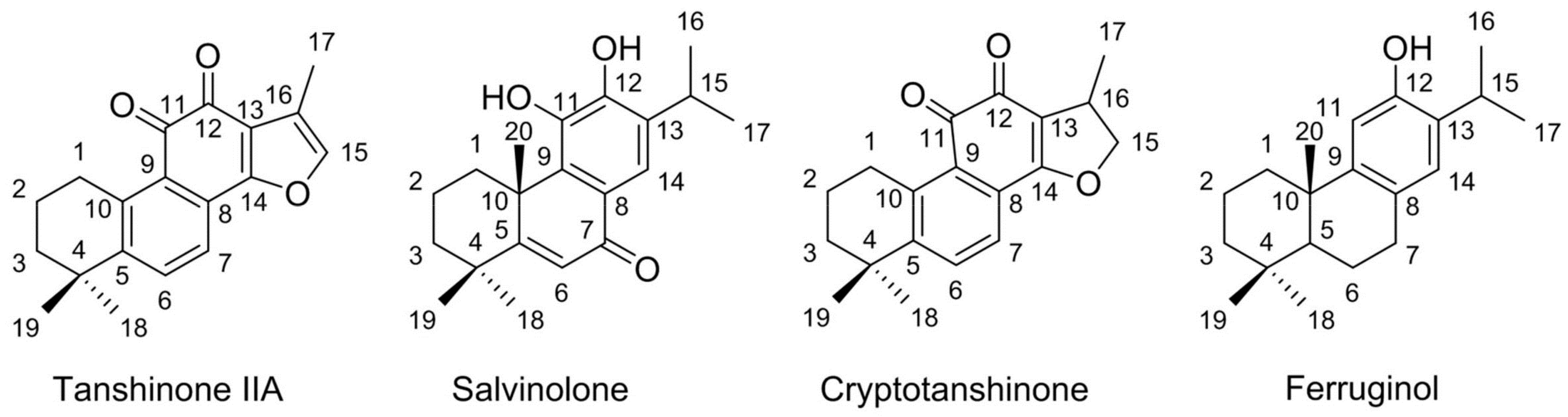
2.7. HPLC Analysis and Identification of the Separated Diterpenes
3. Results and Discussion
3.1. HILIC-SPE Column Chromatography Fractionation and Antibacterial Activity Screening
3.2. Purification of Diterpenes by Reversed-Phase Preparative High-Performance Liquid Chromatography
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- AI-Qudah, M.A.; AI-Jaber, H.I.; Abu Zarga, M.H.; Abu Orabi, S.T. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Cao, B.C.; Yu, A.M.; Zhang, H.Q.; Qiu, F.P. Ultrasound-assisted ionic liquid-based homogeneous liquid-liquid microextraction high-performance liquid chromatography for determination of tanshinones in Salvia miltiorrhiza Bge. root. J. Pharm. Biomed. Anal. 2015, 104, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Shao, Y.; Zhao, J.Q.; Mei, L.J.; Tao, Y.D.; Wang, Q.L.; Zhang, L. Two-dimensional hydrophilic interaction chromatography × reversed-phase liquid chromatography for the preparative isolation of potential anti-hepatitis phenylpropanoids from Salvia prattii. J. Sep. Sci. 2016, 39, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Kitano, Y.; Chiba, K.; Shibata, N.; Kurokawa, H.; Doi, Y.; Arakawa, Y.; Tada, M. Synthesis of variously oxidized abietane diterpenes and their antibacterial activities against MRSA and VRE. Bioorg. Med. Chem. 2001, 9, 347–356. [Google Scholar] [CrossRef]
- Theoduloz, C.; Delporte, C.; Valenzuela-Barra, G.; Silva, X.; Cadiz, S.; Bustamante, F.; Pertino, M.W.; Schmeda-Hirschmann, G. Topical anti-inflammatory activity of new hybrid molecules of terpenes and synthetic drugs. Molecules 2015, 20, 11219–11235. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Liu, L.; Luo, Y.; Odaka, Y.; Awate, S.; Zhou, H.Y.; Shen, T.; Zheng, S.Z.; Lu, Y.; Huang, S.L. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev. Res. 2012, 5, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Su, C.C. Tanshinone IIA may inhibit the growth of small cell lung cancer H146 cells by up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial membrane potential. Mol. Med. Rep. 2010, 3, 645–650. [Google Scholar] [PubMed]
- Yang, J.; Choi, L.L.; Li, D.Q.; Yang, F.Q.; Zeng, L.J.; Zhao, J.; Li, S.P. Simultaneous analysis of hydrophilic and lipophilic compounds in Salvia miltiorrhiza by double-development HPTLC and scanning densitometry. JPC J. Planar Chromat. 2010, 24, 257–263. [Google Scholar] [CrossRef]
- Songsri, S.; Nuntawong, N. Cytotoxic labdane diterpenes from Hedychium ellipticum Buch. -Ham. ex Sm. Molecules 2016, 21, 749. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yang, Z.; Liang, J.L.; Zhou, H.; Wu, S.H. Comprehensive multi-channel multi-dimensional counter-current chromatography for separation of tanshinones from Salvia miltiorrhiza Bunge. J. Chromatogr. A 2014, 1323, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yang, Z.; Liang, J.L.; Guo, M.Z.; Wu, S.H. Multi-channel recycling counter-current chromatography for natural product isolation: Tanshinones as examples. J. Chromatogr. A 2014, 1327, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.L.; Zhang, Y.Q.; Li, A.F.; Meng, Z.L.; Liu, R.M. Extraction and preparative purification of tanshinones from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography. J. Chromatogr. B 2011, 879, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tao, Y.D.; Shao, Y.; Mei, L.J.; Zhang, L.; Wang, Q.L. Antioxidative extracts and phenols isolated from Qinghai-Tibet Plateau medicinal plant Saxifraga tangutica Engl. Ind. Crops Prod. 2015, 78, 13–18. [Google Scholar] [CrossRef]
- Cheng, G.J.S.; Li, G.K.; Xiao, X.H. Microwave-assisted extraction coupled with counter-current chromatography and preparative liquid chromatography for the preparation of six furocoumarins from Angelica pubescentis Radix. Sep. Purif. Technol. 2014, 141, 143–149. [Google Scholar] [CrossRef]
- Ye, X.L.; Cao, D.; Song, F.Y.; Fan, G.R.; Wu, F.H. Preparative separation of nine flavonoids from Pericarpium Citri Reticulatae by preparative-HPLC and HSCCC. Sep. Purif. Technol. 2016, 51, 807–815. [Google Scholar]
- Feng, J.T.; Xiao, Y.S.; Guo, Z.M.; Yu, D.H.; Jin, Y.; Liang, X.M. Purification of compounds from Lignum dalbergia Odorifera using two-dimensional preparative chromatography with Click oligo (ethylene glycol) and C18 column. J. Sep. Sci. 2011, 34, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; He, L.H.; Yang, T. Scale-up purification for rutin hyrdrolysates by high-performance counter-current chromatography coupled with semi-preparative high-performance liquid chromatography. Sep. Purif. Technol. 2016, 51, 1523–1530. [Google Scholar] [CrossRef]
- Zhu, L.C.; Li, H.; Liang, Y.; Wang, X.H.; Xie, H.C.; Zhang, T.Y.; Ito, Y. Application of high-speed counter-current chromatography and preparative high-performance liquid chromatography mode for rapid isolation of anthraquinones from Morinda officinalis How. Sep. Purif. Technol. 2009, 70, 147–152. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, Y.F.; Shen, A.J.; Wang, C.R.; Yan, J.Y.; Zhao, W.J.; Liang, X.M. Efficient purification of active bufadienolides by a class separation method based on hydrophilic solid-phase extraction and reversed-phase high performance liquid chromatography. J. Pharmaceut. Biomed. 2014, 97, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.F.; Jiang, X.H.; Wu, S.H. Direct purification of tanshinones from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography without presaturation of the two-phase solvent mixture. J. Sep. Sci. 2010, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Chen, F. Preparative isolation and purification of six diterpenoids from the Chinese medicinal plant Salvia miltiorrhiza by high-speed counter-current chromatography. J. Chromatogr. A 2001, 925, 109–114. [Google Scholar] [CrossRef]
- Tian, G.L.; Zhang, Y.B.; Zhang, T.Y.; Yang, F.Q.; Ito, Y. Separation of tanshinones from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography using stepwise elution. J. Chromatogr. A 2000, 904, 107–111. [Google Scholar] [CrossRef]
- Wang, J.X.; Guo, Z.M.; Shen, A.J.; Yu, L.; Xiao, Y.S.; Xue, X.Y.; Zhang, X.L.; Liang, X.M. Hydrophilic-subtraction model for the characterization and comparison of hydrophilic interaction liquid chromatography columns. J. Chromatogr. A 2015, 1398, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tao, Y.D.; Wang, D.; Tang, C.C.; Shao, Y.; Wang, Q.L.; Zhao, X.H.; Zhang, Y.Z.; Mei, L.J. A novel two-dimensional preparative chromatography method designed for the separation of traditional animal Tibetan medicine Osteon myospalacem Baileyi. J. Sep. Sci. 2014, 37, 3060–3066. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, J.H.; Kim, Y.M.; Jeong, H.J.; Kim, D.W.; Park, K.H.; Kwon, H.J.; Park, S.J.; Lee, W.S.; Ryu, Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012, 20, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Blasko, G.; Cordell, G.A. Diterpenes of Salvia prionitis. Phytochemistry 1989, 28, 177–181. [Google Scholar] [CrossRef]
- Jang, T.S.; Zhang, H.; Kim, G.; Kim, D.W.; Min, B.S.; Kang, W.; Son, K.H.; Na, M.; Lee, S.H. Bioassay-guided isolation of fatty acid synthase inhibitory diterpenoids from the roots of Salvia miltiorrhiza Bunge. Arch. Pharm. Res. 2012, 35, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Chang, S.T.; Chang, S.C.; Chang, H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008, 22, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the tanshinone IIA, salvinolone, cryptotanshinone and ferruginol are available from the authors. |

| Bacteria | Cf | ||||
|---|---|---|---|---|---|
| Crude diterpene-rich sample | |||||
| Staphylococcus aureus | 125 | 0.5 | |||
| Pseudomonas aeruginosa | 125 | 7.5 | |||
| Acinetobacter baumannii | 250 | 12.5 | |||
| Fractions I, II and III | |||||
| I | II | III | |||
| Staphylococcus aureus | 100 | 125 | 50 | 0.5 | |
| Pseudomonas aeruginosa | 100 | 150 | 50 | 7.5 | |
| Acinetobacter baumannii | 150 | 150 | 50 | 12.5 | |
| Compounds | |||||
| 1 | 2 | 3 | 4 | ||
| Staphylococcus aureus | 25 | 30 | 25 | 10 | 0.5 |
| Pseudomonas aeruginosa | 20 | 50 | 30 | 15 | 7.5 |
| Acinetobacter baumannii | 25 | 50 | 25 | 15 | 12.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, J.; Cui, Y.; Pei, J.; Yue, H.; Liu, Z.; Wang, W.; Jiao, L.; Mei, L.; Wang, Q.; Tao, Y.; et al. Efficient Separation of Four Antibacterial Diterpenes from the Roots of Salvia Prattii Using Non-Aqueous Hydrophilic Solid-Phase Extraction Followed by Preparative High-Performance Liquid Chromatography. Molecules 2018, 23, 623. https://doi.org/10.3390/molecules23030623
Dang J, Cui Y, Pei J, Yue H, Liu Z, Wang W, Jiao L, Mei L, Wang Q, Tao Y, et al. Efficient Separation of Four Antibacterial Diterpenes from the Roots of Salvia Prattii Using Non-Aqueous Hydrophilic Solid-Phase Extraction Followed by Preparative High-Performance Liquid Chromatography. Molecules. 2018; 23(3):623. https://doi.org/10.3390/molecules23030623
Chicago/Turabian StyleDang, Jun, Yulei Cui, Jinjin Pei, Huilan Yue, Zenggen Liu, Weidong Wang, Lijin Jiao, Lijuan Mei, Qilan Wang, Yanduo Tao, and et al. 2018. "Efficient Separation of Four Antibacterial Diterpenes from the Roots of Salvia Prattii Using Non-Aqueous Hydrophilic Solid-Phase Extraction Followed by Preparative High-Performance Liquid Chromatography" Molecules 23, no. 3: 623. https://doi.org/10.3390/molecules23030623





