π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A
Abstract
:1. Introduction
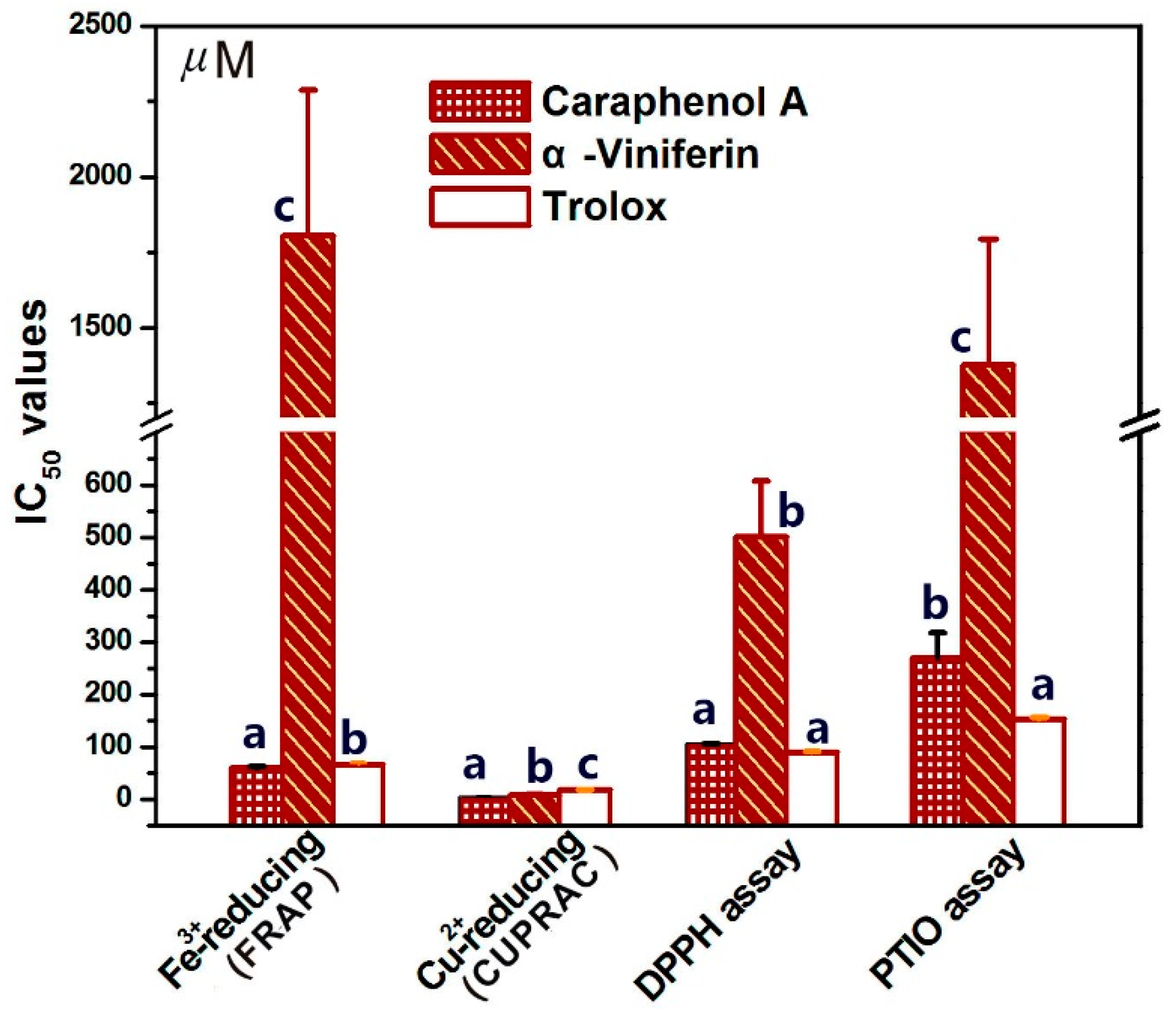
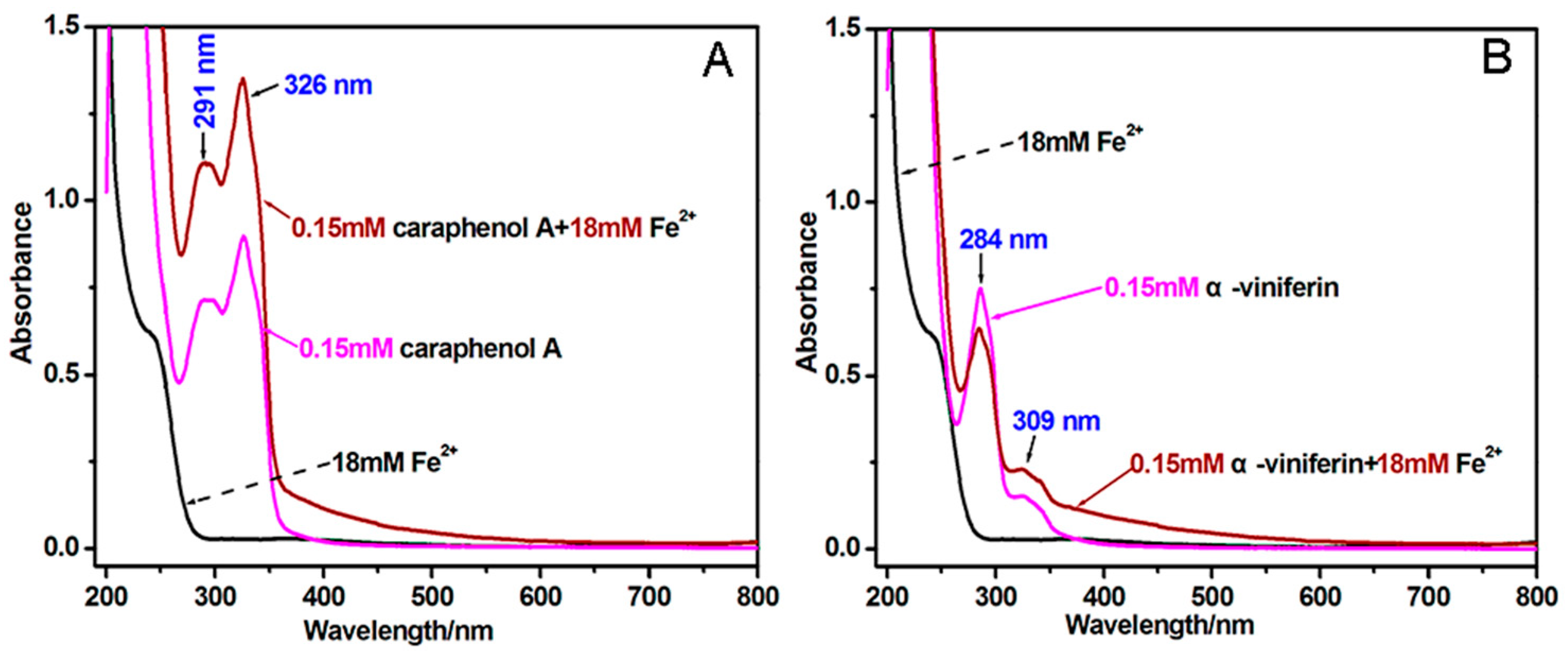
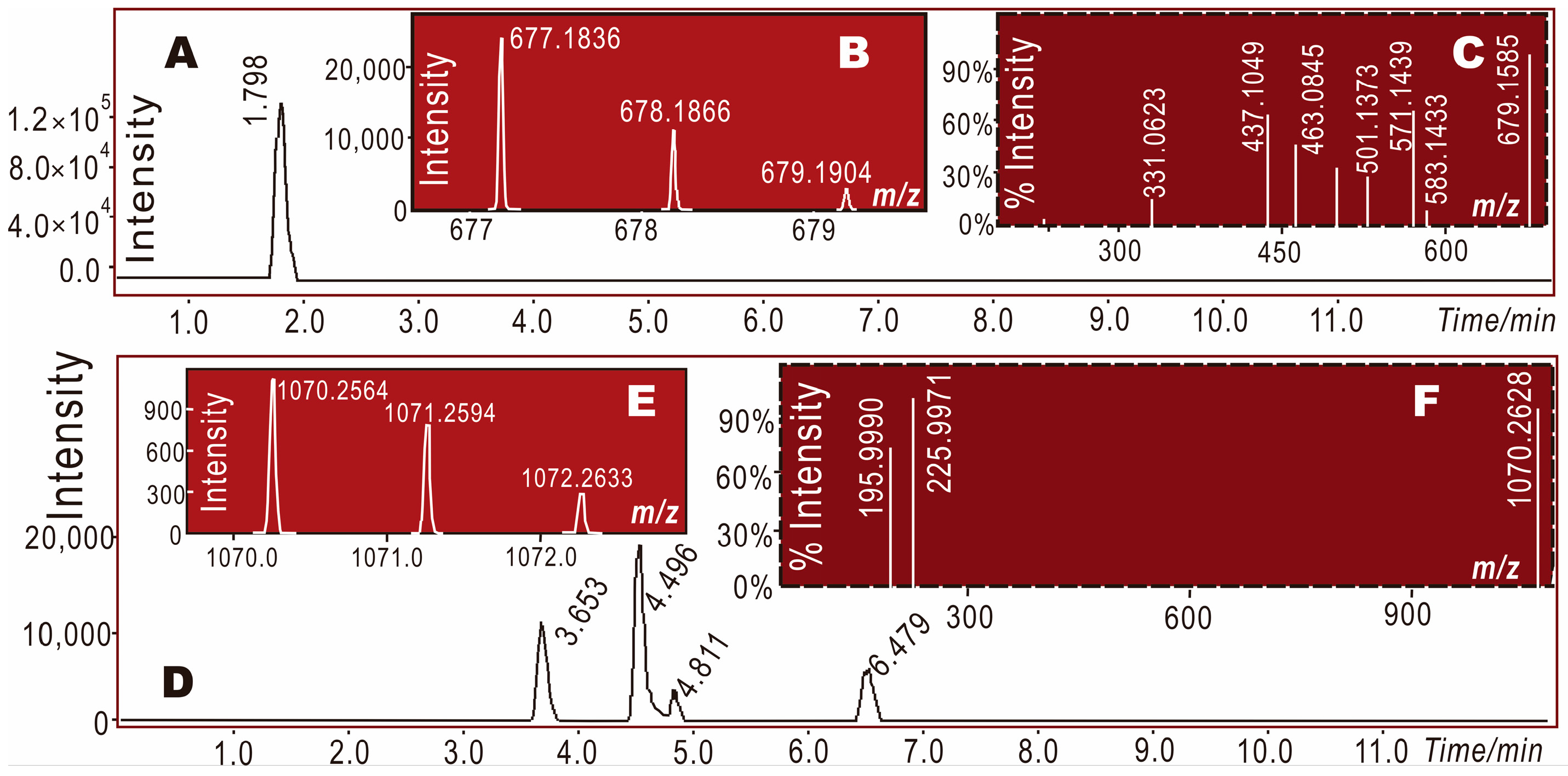
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. FRAP Assay (Fe3+-Reducing Assay)
3.3. CUPRAC Assay (Cu2+-Reducing Assay)
3.4. PTIO•-Scavenging Assay
3.5. DPPH•-Scavenging Assay
3.6. Determining Fe2+-Chelating Ability of α-Viniferin and Caraphenol A (UV-Visible Spectra Analysis)
3.7. Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry (UPLC–ESI–Q–TOF–MS/MS) Determining DPPH• Reaction Products with α-Viniferin or Caraphenol A
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium salt) |
| DPPH• | 1,1-diphenyl-2-picryl-hydrazl |
| ET | electron transfer |
| FRAP | ferric-reducing antioxidant power |
| HAT | hydrogen atom transfer |
| HIV | human immunodeficiency virus |
| ROS | reactive oxygen species |
| RAF | radical adduct formation |
| SD | standard deviation |
| TPTZ | 2,4,6-tris(2-pyridyl-s-triazine) |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid |
References
- Qin, H.L.; Yu, D. 1H-NMR Spectroscopic Databook of Natural Products, 1st ed.; Chemical Industry Press: Beijing, China, 2011; pp. 2224–2235. [Google Scholar]
- Yang, J.S. 13C-NMR Spectroscopic Databook of Natural Products; Chemical Industry Press: Beijing, China, 2011; pp. 2259–2285. [Google Scholar]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6′′-OH group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.F.; Tao, Z.H.; Wang, X.J.; Pan, Y.J. Resveratrol dimers, nutritional components in grape wine, are selective ROS scavengers and weak Nrf2 activators. Food Chem. 2015, 173, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Han, X.H.; Hong, S.S.; Lee, C.; Choe, S.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Antioxidative oligostilbenes from Caragana sinica. Bioorg. Med. Chem. Lett. 2012, 22, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.F.; Zhang, L.P.; Hu, C.Q. Five novel oligostilbenes from the roots of Caragana sinica. Tetrahedron 2001, 57, 4849–4854. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Li, Y.; Zhang, J.; Chen, J.; Lu, W.; Zhao, X.; Lai, Y.; Chen, D.; Wei, G. Protective effect of sinapine against hydroxyl radical-induced damage to mesenchymal stem cells and possible mechanisms. Chem. Pharm. Bull (Tokyo) 2016, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Taverne, Y.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life: A molecular timescale from Earth’s earliest history to the rise of complex life. Bioessays 2018, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Li, X.; Xu, Z.; Liu, X.; Du, S.H.; Li, H.; Zhou, J.H.; Zeng, H.P.; Hua, Z.C. Hexadecanoic acid from Buzhong Yiqi decoction induced proliferation of bone marrow mesenchymal stem cells. J. Med. Food. 2010, 13, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.W.G.; Wang, X.Z.; Liu, D.H.; Deng, R.D.; Li, H.; Zhou, J.H.; Li, Y.W.; Zeng, H.P.; Chen, D.F. Targeting of the Shh pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012, 35, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, X.; Tian, Y.; Lin, Q.; Xie, H.; Lu, W.; Chi, Y.; Chen, D. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect mesenchymal stem cells from •OH-treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant activity/capacity measurement. 1. classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Cekic, S.D.; Baskan, K.S.; Tutem, E.; Apak, R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, W.; Mai, W.; Wang, L. Antioxidant activity and mechanism of Tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013, 8, 787–789. [Google Scholar]
- Li, X.; Gao, Y.; Li, F.; Liang, A.; Xu, Z.; Bai, Y.; Mai, W.; Han, L.; Chen, D. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014, 219, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Rugeles, L.; Alvarez-Idaboy, J.R. A proton-electron sequential transfer mechanism: Theoretical evidence about its biological relevance. Phys. Chem. Chem. Phys. 2015, 17, 28525–28528. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Mai, Y.; Wen, B.; Wang, X. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali (Huangqi). Nat. Prod. Res. 2012, 26, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Saumeth, J.; Rincon, D.A.; Doerr, M.; Daza, M.C. Concerted double proton-transfer electron-transfer between catechol and superoxide radical anion. Phys. Chem. Chem. Phys. 2017, 19, 26179–26190. [Google Scholar] [CrossRef] [PubMed]
- Li, X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) Radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with •NO, •NO2, and •O2−. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.; Malaj, N.; Russo, N.; Toscano, M. Density functional study of the antioxidant activity of some recently synthesized resveratrol analogues. Food Chem. 2013, 141, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.T.; Kim, H.; Thuong, P.T.; Ngoc, T.M.; Lee, I.; Hung, N.D.; Bae, K. Antioxidant and lipoxygenase inhibitory activity of oligostilbenes from the leaf and stem of Vitis amurensis. J. Ethnopharmacol. 2009, 125, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes—A DFT approach. J. Mol. Graph. Model. 2017, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Han, R.M.; Liang, R.; Chen, C.H.; Lai, W.; Zhang, J.P.; Skibsted, L.H. Hydroxyl radical reaction with trans-resveratrol: Initial carbon radical adduct formation followed by rearrangement to phenoxyl radical. J. Phys. Chem. B 2012, 116, 7154–7161. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Lin, J.; Wang, T.; Huang, J.; Lin, Y.; Chen, D. Protective effects of dihydromyricetin against •OH-Induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Galano, A.; Vargas, R. Free radical scavenger properties of alpha-mangostin: Thermodynamics and kinetics of HAT and RAF mechanisms. J. Phys. Chem. B 2011, 115, 12591–12598. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Liu, J.; Liu, Y.; Zhang, J.; Lin, J.; Zhao, Z.; Chen, D. Effect and mechanism of wedelolactone as antioxidant-coumestan on •OH-treated mesenchymal stem cells. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Xie, H.; Li, X.; Ren, Z.; Qiu, W.; Chen, J.; Jiang, Q.; Chen, B.; Chen, D. Antioxidant and cytoprotective effects of Tibetan tea and its phenolic components. Molecules 2018, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.E.; Iuga, C.; Alvarez-Idaboy, J.R. Antioxidant activity of propyl gallate in aqueous and lipid media: A theoretical study. Phys. Chem. Chem. Phys. 2013, 15, 13137–13146. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Hu, Q.P.; Jiang, S.X.; Li, F.; Lin, J.; Han, L.; Hong, Y.L.; Lu, W.B.; Gao, Y.X.; Chen, D.F. Flos Chrysanthemi Indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism. J. Saudi Chem. Soc. 2015, 19, 454–460. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zeng, H. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Acta Chim. Sin. 2009, 67, 974–982. [Google Scholar]
- Wang, T.T.; Zeng, G.C.; Li, X.C.; Zeng, H.P. In vitro studies on the antioxidant and protective effect of 2-substituted -8-hydroxyquinoline derivatives against H2O2-induced oxidative stress in BMSCs. Chem. Biol. Drug Des. 2010, 75, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Lin, J.; Li, Y.; Wang, T.; Jiang, Q.; Chen, D. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: A bioevaluation and mechanistic chemistry. BMC Complement. Altern. Med. 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Mai, W.Q.; Chen, D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
Sample Availability: Samples of the α-viniferin is available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xie, Y.; Xie, H.; Yang, J.; Chen, D. π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules 2018, 23, 694. https://doi.org/10.3390/molecules23030694
Li X, Xie Y, Xie H, Yang J, Chen D. π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules. 2018; 23(3):694. https://doi.org/10.3390/molecules23030694
Chicago/Turabian StyleLi, Xican, Yulu Xie, Hong Xie, Jian Yang, and Dongfeng Chen. 2018. "π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A" Molecules 23, no. 3: 694. https://doi.org/10.3390/molecules23030694




