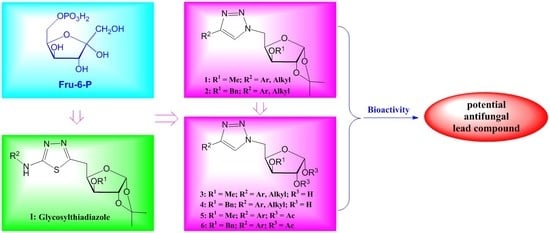
3.2. Chemical Synthesis
General procedure for the synthesis of title compounds 1/
2. To a soln of compound
7 [
22] or
8 [
23] (1.5 g) in 1:1:1 CH
2Cl
2–CH
3OH–H
2O (30 mL) was added alkyne derivaticves (0.35 mL), CuSO
4·5H
2O (0.45 g) and sodium ascorbate (0.315 g). The mixture was stirred at 40 °C for 10 h, and TLC (6:1 petroleum ether–EtOAc) indicated that the reaction was complete. The aq. soln. was extracted with CH
2Cl
2 (3 × 50 mL), washed with saturated aq. sodium bicarbonate, dried (Na
2SO
4) and concentrated. Purification by silica gel chromatography with 7:1 petroleum ether–EtOAc as the eluent afforded
1 or
2.
General procedure for the synthesis of title compounds 3/4. Compound 1 or 2 (0.8 g) was dissolved in 90% aq trifluoroacetic acid (20 mL) and then stirred at 40 °C for 4 h, and TLC (1:1 petroleum ether–EtOAc) indicated that the reaction was complete. The trifluoroacetic acid was evaporated under reduced pressure, then the residue was diluted with CH2Cl2 (50 mL), washed with saturated aq. sodium bicarbonate, and dried over Na2SO4. The soln was concentrated, and the residue was subjected to column chromatography (2:1 petroleum ether–EtOAc) to give the desired product 3/4.
General procedure for the synthesis of title compounds 5/6. To a stirred of compound 3 or 4 (0.4 g) in pyridine (5 mL) was added acetic anhydride (3 mL). The mixture was stirred for a further 3 h, at the end of which time TLC (eluent: 4:1 petroleum ether–EtOAc) indicated that the reaction was complete. The solvents were evaporated under reduced pressure to give a crude product, which was purified on silica gel column chromatography with 5:1 petroleum ether–EtOAc as the eluent to give the compounds 5/6.
Furan glucosyl-1,2,3-triazole (1-a). Yield: 79.4%. White solid, m.p. 159.9–160.4 °C. 1H-NMR (CDCl3): δ 7.89–7.31 (m, 6H, ArH, CCHN), 5.95 (s, 1H, H-1), 4.77–3.76 (m, 5H, H-2, H-3, H-4, H-5, H-6), 3.44 (s, 3H, CH3O), 1.43, 1.30 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 147.44, 130.53, 128.56, 127.81, 125.48, 120.69, 111.74, 105.01, 83.76, 81.16, 78.65, 57.53, 48.82, 26.52, 25.98. ESI-MS m/z calcd. for C17H22O4N3 [M + H]+ 332.1. Found: 332.1. HRMS for C17H22O4N3 [M + H]+ 332.1610. Found: 332.1602.
Furan glucosyl-1,2,3-triazole (1-b). Yield: 79.6%. Yellow solid, m.p. 108.0–112.7 °C. 1H-NMR (CDCl3): δ 7.88–7.12 (m, 5H, ArH, CCHN), 5.95 (s, 1H, H-1), 4.76–3.75 (m, 5H, H-2, H-3, H-4, H-5, H-6), 3.43 (s, 3H, CH3O), 2.38 (s, 3H, Ar-Me), 1.43, 1.30 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 138.34, 130.51, 128.77, 128.61, 126.33, 122.78, 120.71, 111.98, 105.17, 83.97, 81.32, 78.82, 57.73, 48.98, 26.68, 26.14, 21.32. ESI-MS m/z calcd. for C18H24O4N3 [M + H]+ 347.1. Found: 347.1. HRMS for C18H24O4N3 [M + H]+: 347.1719. Found: 347.1718.
Furan glucosyl-1,2,3-triazole (1-c). Yield: 69.3%. White solid, m.p. 156.6–157.5 °C. 1H-NMR (CDCl3): δ 7.82–6.93 (m, 5H, ArH, CCHN), 5.96 (d, 1H, J = 4.0 Hz, H-1), 4.77–4.50 (m, 4H), 3.83–3.76 (m, 4H), 3.44 (s, 3H, CH3O) 1.43, 1.32 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 159.47, 147.49, 126.90, 123.35, 119.92, 114.12, 111.89, 105.12, 83.91, 81.28, 78.79, 57.67, 55.16, 48.88, 26.63, 26.09. ESI-MS m/z calcd. for C18H24O5N3 [M + H]+ 362.1. Found: 362.1. HRMS for C18H24O5N3 [M + H]+ 362.1716. Found: 362.1710.
Furan glucosyl-1,2,3-triazole (1-d). Yield: 83.0%. White solid, m.p. 121.4–122.3 °C. 1H-NMR (CDCl3): δ 7.88–7.08 (m, 5H, ArH, CCHN), 5.97 (s, 1H, H-1), 4.78–3.79 (m, 5H, H-2, H-3, H-4, H-5, H-6), 3.45 (s, 3H, CH3O), 1.43, 1.31 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 163.77, 161.31, 146.83, 127.45, 127.37, 127.07, 126.97, 126.94, 120.57, 115.77, 115.55, 112.00, 105.19, 84.03, 81.33, 78.82, 77.48, 57.73, 49.12, 26.27, 26.12. ESI-MS m/z calcd. for C17H21O4N3F [M + H]+ 350.1. Found: 350.1. HRMS for C17H21O4N3F [M + H]+ 350.1516. Found: 350.1515.
Furan glucosyl-1,2,3-triazole (1-e). Yield: 77.1%. White solid, m.p. 126.5–126.8 °C. 1H-NMR (CDCl3): δ 8.28–8.01 (m, 5H, ArH, CCHN), 5.99 (s, 1H, H-1), 4.84–3.85 (m, 5H, H-2, H-3, H-4, H-5, H-6), 3.49 (s, 3H, CH3O), 1.44, 1.33 (2s, 6H, Me2C); 13C-NMR (CDCl3) δ: 147.25, 145.61, 137.04, 126.15, 124.22, 122.49, 112.13, 105.26, 84.16, 81.38, 78.77, 57.85, 49.59, 26.71, 26.15. ESI-MS m/z calcd. for C17H21O6N4 [M + H]+ 377.1. Found: 377.1. HRMS for C17H21O6N4 [M + H]+ 377.1461. Found: 377.1461.
Furan glucosyl-1,2,3-triazole (1-f). Yield: 79.3%. White solid, m.p. 135.9–136.7 °C. 1H-NMR (CDCl3): δ 7.90–7.37 (m, 5H, ArH, CCHN), 5.97 (s, 1H, H-1), 4.78–3.79 (m, 5H, H-2, H-3, H-4, H-5, H-6) 3.46 (s, 3H, CH3O) 1.43, 1.31 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 146.74, 133.75, 129.27, 128.98, 127.00, 120.93, 112.10, 105.25, 84.11, 81.38, 78.85, 57.83, 49.27, 26.74, 26.20. ESI-MS m/z calcd. for C17H21O4N3Cl [M + H]+ 366.1. Found: 366.1. HRMS for C17H21O4N3Cl [M + H]+ 366.1221. Found: 366.1219.
Furan glucosyl-1,2,3-triazole (1-g). Yield: 92.3%. Yellow solid, m.p. 90.2–90.9 °C. 1H-NMR (CDCl3): δ 7.61 (d, J = 4.0 Hz, 1H, CCHN), 5.94 (s, 1H, H-1), 5.09–5.04 (m, 1H, CH3CHOH), 4.68–4.47 (m, 4H), 3.75 (s, 1H), 3.44 (s, 3H, CH3O), 1.58 (s, 3H, OH-C-CH3)1.43,1.30 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 162.75, 152.52, 152.46, 121.43, 112.08, 105.21, 84.00, 81.36, 78.80, 62.83, 62.77, 57.79, 48.99, 29.67, 26.73, 26.19, 23.09, 23.00, 20.96. ESI-MS m/z calcd. for C13H23O5N3 [M + H]+ 300.1. Found: 300.1. HRMS for C13H23O5N3 [M + H]+ 300.1559. Found: 300.1555.
Furan glucosyl-1,2,3-triazole (
2-a) [
24]. Yield: 66.0%. White solid, m.p. 133.2–134.1 °C.
1H-NMR (CDCl
3): δ 7.79–7.25(m, 11H, ArH, CCHN), 5.96 (s, 1H, H-1), 4.69–4.42 (m, 6H, H-2, H-3, H-4, H-5,
CH
2Ar), 3.97 (s, 1H, H-6), 1.40, 1.27 (2s, 6H, Me
2C);
13C-NMR (CDCl
3): δ 147.53, 136.84, 128.60, 128.52, 128.13, 127.86, 127.79, 125.57, 120.73, 111.92, 111.90, 81.86, 81.61, 78.76, 77.48, 76.84, 76.81, 71.87, 49.09, 26.62, 26.08. ESI-MS
m/z calcd. for C
23H
26O
4N
3 [M + H]
+ 408.1 Found: 408.1. HRMS for C
23H
26O
4N
3 [M + H]
+ 408.1923. Found: 408.1922.
Furan glucosyl-1,2,3-triazole (2-b). Yield: 79.4%. White solid, m.p. 108.8–110.2 °C. 1H-NMR (CDCl3): δ 7.77–7.09 (m, 11H, ArH, CCHN), 5.98 (s, 1H, H-1), 4.72–4.44 (m, 6H, H-2, H-3, H-4, H-5, CH2Ar), 3.98 (s, 1H, H-6), 2.36 (s, 3H, CH3Ar), 1.41,1.29 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 147.72, 138.24, 136.92, 130.51, 128.69, 128.57, 128.17, 127.83, 126.30, 122.77, 120.68, 111.98, 105.18, 81.96, 81.74, 78.84, 71.96, 49.12, 26.67, 26.14, 21.27. ESI-MS m/z calcd. for C24H28O4N3 [M + H]+ 422.2. Found: 422.2. HRMS for C24H28O4N3 [M + H]+ 422.2080. Found: 422.2078.
Furan glucosyl-1,2,3-triazole (2-c). Yield: 73.6%. White solid, m.p. 134.6–135.1 °C. 1H-NMR (CDCl3): δ 7.73–6.92 (m, 11H, ArH, CCHN), 6.00 (s, 1H, H-1), 4.75–4.48 (m, 6H, H-2, H-3, H-4, H-5, CH2Ar), 4.01–3.81 (m, 4H, CH3O, H-6), 1.42, 1.30 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 159.58, 147.64, 136.96, 128.71, 128.32, 127.96, 127.04, 123.42, 120.03, 114.21, 112.13, 105.28, 82.03, 81.78, 78.97, 72.07, 55.29, 49.23, 26.77, 26.24. ESI-MS m/z calcd. for C24H28O5N3 [M + H]+ 438.2. Found: 438.2. HRMS for C24H28O5N3 [M + H]+ 438.2029. Found: 438.2044.
Furan glucosyl-1,2,3-triazole (2-d). Yield: 90.4%. White solid, m.p. 164.3–164.7 °C. 1H-NMR (CDCl3): δ 7.77–7.06 (m, 10H, ArH, CCHN), 6.00 (s, 1H, H-1), 4.75–4.48 (m, 6H, H-2, H-3, H-4, H-5, CH2Ar), 4.02 (s, 1H, H-6), 1.42, 1.31 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 146.92, 136.95, 128.73, 128.36, 127.98, 127.53, 127.45, 126.99, 120.63, 115.82, 115.61, 112.18, 105.31, 82.07, 81.83, 78.94, 72.09, 49.40, 26.77, 26.24. ESI-MS m/z calcd. for C23H25O4N3F [M + H]+ 426.1. Found: 426.1. HRMS for C23H25O4N3F [M + H]+ 426.1829. Found: 426.1830.
Furan glucosyl-1,2,3-triazole (2-e). Yield: 90.2%. White solid, m.p. 182.6–184.6 °C. 1H-NMR (CDCl3): δ 8.28–7.33 (m, 11H, ArH, CCHN), 6.03 (s, 1H, H-1), 4.80–4.50 (m, 6H, H-2, H-3, H-4, H-5, CH2Ar), 4.07 (s, 1H, H-6), 1.43, 1.33 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 147.33, 145.67, 137.02, 136.90, 128.81, 128.46, 128.03, 126.21, 124.27, 122.51, 112.29, 105.37, 82.08, 81.83, 78.84, 72.12, 49.80, 26.79, 26.25. ESI-MS m/z calcd. for C23H24O6N4 [M + H] 453.1. Found: 453.1. HRMS for C23H24O6N4 [M + H]+ 453.1774. Found: 453.1773.
Furan glucosyl-1,2,3-triazole (2-f). Yield: 93.7%. White solid, m.p. 136.2–136.5 °C. 1H-NMR (CDCl3): δ 7.79–7.33 (m, 11H, ArH, CCHN), 6.01 (s, 1H, H-1), 4.77–4.48 (m, 6H, H-2, H-3, H-4, H-5, CH2Ar), 4.03 (s, 1H, H-6), 1.43, 1.31 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 146.80, 136.94, 133.82, 129.28, 129.02, 128.80, 128.44, 128.03, 127.06, 120.98, 112.24, 105.35, 82.08, 81.82, 78.95, 77.16, 76.84, 72.13, 49.51, 26.82, 26.29. ESI-MS m/z calcd. for C23H25O4N3Cl [M + H]+ 442.1. Found: 442.1. HRMS for C23H25O4N3Cl [M + H]+ 442.1534. Found: 442.1533.
Furan glucosyl-1,2,3-triazole (2-g). Yield: 87.6%. White solid, m.p. 90.6–90.9 °C. 1H-NMR (CDCl3): δ 7.46–7.23 (m, 6H, ArH, CCHN), 5.86 (s, 1H, H-1), 4.93–4.92 (s, 1H), 4.63–4.39 (m, 6H, H-2, H-3, H-4, H-5, CH2Ar), 3.89 (s, 1H, H-6), 1.45 (d, J = 4.0 Hz, 3H, OH-C-CH3) 1.31,1.20 (2s, 6H, Me2C); 13C-NMR (CDCl3): δ 136.84, 128.51, 128.11, 127.79, 121.33, 111.90, 105.06, 81.87, 81.57, 78.73, 71.87, 62.62, 62.57, 48.99, 26.57, 26.05, 23.04, 22.96. ESI-MS m/z calcd. for C19H27O5N3 [M + H]+ 376.1. Found: 376.1. HRMS for C19H27O5N3 [M + H]+ 376.1872. Found: 376.1875.
Furan glucosyl-1,2,3-triazole (3-a). Yield: 78.5%. White solid, m.p. 101.1–102.1 °C. 1H-NMR (Meth-d4): δ 8.18–7.21 (m, 5H, ArH, CCHN), 5.26–5.08 (m, 1H, H-1, α and β), 4.82–3.71 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.36 (m, 3H, OMe); 13C-NMR (Meth-d4): δ 148.60, 148.54, 131.65, 131.63, 129.92, 129.27, 126.63, 123.26, 123.09, 104.80, 97.85, 87.13, 86.70, 80.54, 79.71, 77.50, 76.00, 58.42, 52.55, 51.73. ESI-MS m/z calcd. for C14H18O4N3 [M + H]+ 292.1. Found: 292.1. HRMS for C14H18O4N3 [M + H]+ 292.1297. Found: 292.1293.
Furan glucosyl-1,2,3-triazole (3-b). Yield: 77.4%. Oily. 1H-NMR (Meth-d4): δ 8.19–6.98 (m, 5H, ArH, CCHN), 5.27–5.10 (m, 1H, H-1, α and β), 4.60–3.69 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.32 (s, 3H, CH3O), 2.20 (s, 3H, Ar-CH3); 13C-NMR (Meth-d4): δ 148.52, 148.45, 139.68, 131.23, 130.00, 129.80, 127.16, 123.74, 123.24, 123.04, 104.73, 97.80, 87.02, 86.61, 80.44, 79.61, 77.43, 75.84, 58.41, 58.31, 52.55, 51.71, 21.44. ESI-MS m/z calcd. for C15H20O4N3 [M + H]+ 306.1. Found: 306.1. HRMS for C15H20O4N3 [M + H]+ 306.1454. Found: 306.1456.
Furan glucosyl-1,2,3-triazole (3-c). Yield: 79.4%. White solid, m.p. 130.3–130.8 °C. 1H-NMR (Meth-d4): δ 8.06–6.83 (m, 5H, ArH, CCHN), 5.26–5.07 (m, 1H, H-1, α and β), 4.79–3.98 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.78–3.76 (m, 3H, CH3O-Ar), 3.20 (m, 3H, OMe); 13C-NMR (Meth-d4): δ 160.20, 148.54, 148.48, 127.97, 124.22, 124.19, 122.39, 122.22, 115.33, 104.79, 97.84, 87.11, 86.69, 80.55, 79.69, 77.52, 75.98, 58.41, 58.34, 55.77, 52.47, 51.66. ESI-MS m/z calcd. for C15H30O5N3 [M + H]+ 322.1. Found: 322.1. HRMS for C15H30O5N3 [M + H]+ 322.1403. Found: 322.1399.
Furan glucosyl-1,2,3-triazole (3-d). Yield: 64.9%. White solid, m.p. 136.5–137.1 °C. 1H-NMR (Meth-d4): δ 8.15–7.00 (m, 5H, ArH, CCHN), 5.27–5.08 (m, 1H, H-1, α and β), 4.76–4.50 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.45–3.20 (m, 3H, OMe); 13C-NMR (Meth-d4): δ 163.91, 161.47, 146.40, 146.33, 127.29, 127.21, 126.85, 126.81, 126.78, 121.78, 121.62, 115.48, 115.27, 103.49, 96.55, 85.80, 85.39, 79.20, 78.39, 76.20, 74.66, 57.11, 57.03, 51.21, 50.40. ESI-MS m/z calcd. for C14H17O4N3F [M + H]+ 310.1. Found: 310.1. HRMS for C14H17O4N3F [M + H]+ 310.1203. Found: 310.1204.
Furan glucosyl-1,2,3-triazole (3-e). Yield: 74.3%. White solid, m.p. 149.2–150.1 °C. 1H-NMR (DMSO): δ 8.81–8.14 (m, 5H, ArH, CCHN), 6.19–6.11 (m, 1H, H-1, α and β), 5.00–4.56 (m, 4H), 4.06–3.99 (m, 1H), 3.44–3.37 (m, 3H, OMe); 13C-NMR (DMSO): δ 146.69, 146.59, 144.42, 144.20, 137.05, 137.05, 125.98, 125.86, 124.35, 103.29, 96.29, 85.92, 85.07, 78.89, 77.73, 75.39, 74.31, 57.50, 57.44, 51.06, 50.39. ESI-MS m/z calcd. for C14H17O6N4 [M + H]+ 337.1. Found: 337.1. HRMS for C14H17O6N4 [M + H]+ 337.1148. Found: 337.1146.
Furan glucosyl-1,2,3-triazole (3-f). Yield: 80.7%. White solid, m.p. 109.4–110.2 °C. 1H-NMR (Meth-d4): δ 8.19–7.25 (m, 5H, ArH, CCHN), 5.29–5.10 (m, 1H, H-1, α and β), 5.07–3.72 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.46–3.21 (m, 3H, OMe); 13C-NMR (Meth-d4): δ 133.62, 129.00, 128.98, 128.74, 126.75, 122.20, 122.01, 103.46, 96.56, 85.76, 85.37, 79.21, 78.32, 76.22, 74.59, 57.18, 57.08, 51.32, 50.48. ESI-MS m/z calcd. for C14H17O4N3Cl [M + H]+ 326.0. Found: 326.0. HRMS for C14H17O4N3Cl [M + H]+ 326.0908. Found: 326.0906.
Furan glucosyl-1,2,3-triazole (3-g). Yield: 79.1%. Oily. 1H-NMR (Meth-d4): δ 7.86–7.83 (m, 1H, CCHN), 5.25–5.05 (m, 1H, H-1, α and β),4.97–4.46 (m, 4H), 4.05–3.69 (m, 2H), 3.36–3.33 (m, 3H, OMe), 1.44–1.42 (m, 3H, CH-CH3); 13C-NMR (Meth-d4): δ 153.54, 153.49, 123.81, 123.59, 104.76, 97.86, 87.01, 86.63, 80.50, 80.39, 78.80, 79.55, 77.47, 75.84, 63.41, 58.39, 58.31, 58.11, 52.68, 51.82, 23.63. ESI-MS m/z calcd. for C10H18O5N3 [M + H]+ 260.1. Found: 260.1. HRMS for C10H18O5N3 [M + H]+ 260.1403. Found: 260.1247.
Furan glucosyl-1,2,3-triazole (4-a). Yield: 80.3%. White solid, m.p. 98.7–99.2 °C. 1H-NMR (Meth-d4): δ 8.07–7.14 (m, 11H, ArH, CCHN), 5.29–5.10 (m, 1H, H-1, α and β), 4.77–3.92 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β); 13C-NMR (Meth-d4): δ 148.52, 148.46, 139.16, 139.00, 131.65, 131.61, 129.89, 129.47, 129.46, 129.23, 129.10, 129.01, 128.91, 128.88, 126.61, 123.28, 123.11, 104.79, 97.85, 84.96, 84.56, 80.37, 77.46, 76.44, 73.24, 73.15, 52.65, 51.84. ESI-MS m/z calcd. for C20H22O4N3 [M + H]+ 368.1. Found: 368.1. HRMS for C20H22O4N3 [M + H]+ 368.1610. Found: 368.1608.
Furan glucosyl-1,2,3-triazole (4-b). Yield: 74.2%. White solid, m.p. 90.2–90.6 °C. 1H-NMR (Meth-d4): δ 8.03–6.98 (m, 10H, ArH, CCHN), 5.28–5.10 (m, 1H, H-1, α and β), 4.75–3.91 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β), 2.22 (s, 3H, Ar-Me); 13C-NMR (Meth-d4): δ 147.33, 147.27, 138.38, 137.87, 137.71, 130.20, 128.63, 128.50, 128.17, 128.16, 127.79, 127.70, 127.60, 127.58, 125.88, 122.46, 121.94, 121.75, 103.49, 96.55, 83.68, 83.28, 79.07, 76.16, 75.14, 71.94, 71.84, 51.32, 50.51, 20.17. ESI-MS m/z calcd. for C23H22O4N3 [M + H]+ 382.1. Found: 382.1. HRMS for C23H22O4N3 [M + H]+ 382.1767. Found: 382.1766.
Furan glucosyl-1,2,3-triazole (4-c). Yield: 76.8%. Yellow solid, m.p. 121.6–123.0 °C. 1H-NMR (Meth-d4): δ 7.98–6.79 (m, 10H, ArH, CCHN), 5.30–5.11 (m, 1H, H-1, α and β), 4.60–3.92 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β), 3.63(s, 3H, Ar-O-Me); 13C-NMR (Meth-d4): δ 161.23, 139.11, 138.96, 129.45, 129.06, 128.97, 128.89, 128.87, 128.02, 122.42, 115.34, 104.78, 97.88, 84.94, 84.57, 80.31, 77.44, 76.32, 73.22, 73.12, 55.77, 52.76, 51.93. ESI-MS m/z calcd. for C21H24O5N3 [M + H]+ 398.1. Found: 398.1. HRMS for C21H24O5N3 [M + H]+ 398.1716. Found: 398.1715.
Furan glucosyl-1,2,3-triazole (4-d). Yield: 80.5%.White solid, m.p. 132.7–134.2 °C. 1H-NMR (Meth-d4): δ 8.07–6.98 (m, 10H, ArH, CCHN), 5.30–5.10 (m, 1H, H-1, α and β), 5.02–4.04 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β); 13C-NMR (Meth-d4): δ 164.27, 162.82, 139.13, 138.97, 129.48, 129.47, 129.11, 129.01, 128.64, 123.29, 123.10, 116.82, 116.60, 104.80, 97.90, 84.95, 84.57, 80.35, 77.46, 76.38, 73.27, 73.17, 52.78, 51.96. ESI-MS m/z calcd. for C20H21O4N3F [M + H]+ 386.1. Found: 386.1. HRMS for C20H21O4N3F [M + H]+ 386.1516. Found: 386.1515.
Furan glucosyl-1,2,3-triazole (4-e). Yield: 81.6%. White solid, m.p. 156.2–156.9 °C. 1H-NMR (DMSO): δ 8.80–7.30 (m, 10H, ArH, CCHN), 6.56–6.33 (m, 1H, H-1, α and β), 4.76–4.01 (m, 9H); 13C-NMR (DMSO): δ 146.54, 144.21, 138.09, 137.18, 128.26, 127.57, 127.53, 125.82, 124.32, 124.11, 96.30, 96.20, 83.10, 75.34, 74.71, 71.17, 50.53. ESI-MS m/z calcd. for C20H21O6N4 [M + H]+ 413.1. Found: 413.1. HRMS for C20H21O6N4 [M + H]+ 413.1461. Found: 413.1460.
Furan glucosyl-1,2,3-triazole (4-f).Yield: 73.4%. White solid, m.p. 103.7–104.4 °C. 1H-NMR (Meth-d4): δ 8.08–7.15 (m, 10H, ArH, CCHN), 5.29–5.09 (m, 1H, H-1, α and β), 4.74–3.92 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β); 13C-NMR (Meth-d4): δ 147.39, 147.32, 139.17, 139.01, 134.86, 130.01, 129.48, 129.47, 128.03, 123.49, 123.32, 104.80, 97.86, 84.99, 84.58, 80.39, 80.34, 77.44, 76.45, 73.26, 73.16, 52.71, 51.90. ESI-MS m/z calcd. for C20H21O4N3Cl [M + H]+ 367.1. Found: 367.1. HRMS for C20H21O4N3Cl [M + H]+ 402.1221. Found: 402.1220.
Furan glucosyl-1,2,3-triazole (4-g). Yield: 79.7%. White solid, m.p. 105.7–106.1 °C. 1H-NMR (Meth-d4): δ 7.75–7.14 (m, 6H, ArH, CCHN), 5.28–5.08 (m, 1H, H-1, α and β), 4.64–4.05 (m, 8H), 1.59–1.56 (m, 3H, CH-CH3); 13C-NMR (Meth-d4): δ 153.35, 153.26, 139.08, 138.93, 129.41, 128.99, 128.91, 128.89, 123.61, 123.41, 104.65, 97.75, 84.80, 84.78, 84.47, 80.38, 80.20, 77.45, 76.20, 73.13, 73.06, 63.47, 52.52, 51.68, 23.61. ESI-MS m/z calcd. for C16H22O5N3 [M + H]+ 353.1. Found: 353.1. HRMS for C16H22O5N3 [M + H]+ 353.1907. Found: 353.1906.
Furan glucosyl-1,2,3-triazole (5-a). Yield: 88.2%. White solid, m.p. 140.2–141 °C. 1H-NMR (CDCl3): δ 7.87–7.26 (m, 6H, ArH, CCHN), 6.45–6.14 (m, 1H, H-1, α and β), 5.26–4.42 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β) 3.51–3.45 (m, 3H, OMe, α and β) 2.14–2.03 (m, 6H, Me2-CO); 13C-NMR (CDCl3): δ 169.61, 169.27, 147.96, 130.62, 128.93, 128.27, 127.70, 125.86, 121.15, 120.89, 99.80, 94.06, 82.77, 82.40, 81.79, 78.62, 78.25, 76.16, 58.45, 58.27, 50.36, 49.88, 21.27, 20.91, 20.82, 20.54. ESI-MS m/z calcd. for C18H22O6N3 [M + H]+ 376.1. Found: 376.1. HRMS for C18H22O6N3 [M + H]+ 376.1509. Found: 376.1507.
Furan glucosyl-1,2,3-triazole (5-b). Yield: 79.4%. White solid, m.p. 116.7–118.1 °C. 1H-NMR (CDCl3): δ 7.78–7.04 (m, 5H, ArH, CCHN), 6.38–6.08 (m, 1H, H-1, α and β), 5.19–3.79 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β) 3.42–3.37 (m, 3H, OMe, α and β) 2.30 (s, 3H, Ar-CH3) 2.06–1.95 (m, 6H, Me2-CO, α and β); 13C-NMR (CDCl3): δ 169.52, 169.16, 147.90, 138.46, 130.42, 128.90, 128.71, 126.39, 122.85, 121.04, 120.73, 99.67, 93.93, 82.63, 82.26, 81.72, 78.51, 78.13, 76.09, 58.32, 58.15, 50.22, 49.73, 21.39, 21.15, 20.79, 20.69, 20.43. ESI-MS m/z calcd. for C19H24O6N3 [M + H]+ 390.1. Found: 390.1. HRMS for C19H24O6N3 [M + H]+ 390.1665. Found: 390.1668.
Furan glucosyl-1,2,3-triazole (5-c). Yield: 91.9%. White solid, m.p. 156.7–157.9 °C. 1H-NMR (CDCl3): δ 7.78–6.93 (m, 5H, ArH, CCHN), 6.45–6.15 (m, 1H, H-1, α and β), 5.26–4.40 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.82 (m, 3H, Ar-OMe), 3.50–3.45 (m, 3H, OMe), 2.14–2.03 (m, 6H, Me2-CO, α and β); 13C-NMR (CDCl3): δ 169.61, 169.26, 159.74, 147.84, 127.16, 123.40, 120.31, 120.04, 114.36, 99.81, 94.10, 82.74, 82.44, 81.85, 78.62, 78.33, 76.16, 58.43, 58.25, 55.41, 50.25, 49.79, 21.26, 20.90, 20.81, 20.54. ESI-MS m/z calcd. for C18H21O7N3 [M + H]+ 406.1. Found: 406.1. HRMS for C18H21O7N3 [M + H]+ 406.1614. Found: 406.1612.
Furan glucosyl-1,2,3-triazole (5-d). Yield: 90.7%. White solid, m.p. 141.6–143.6 °C. 1H-NMR (CDCl3): δ 7.78–7.00 (m, 5H, ArH, CCHN), 6.38–6.08 (m, 1H, H-1, α and β), 5.20–3.81 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.44–3.38 (m, 3H, OMe), 2.07–1.96 (m, 6H, Me2-CO, α and β); 13C-NMR (CDCl3): δ 169.48, 169.43, 169.12, 163.85, 161.39, 146.92, 127.49, 127.41, 120.83, 120.53, 115.84, 115.62, 99.66, 93.88, 82.65, 82.22, 81.66, 78.49, 78.04, 76.06, 58.30, 58.14, 50.32, 49.82, 21.10, 20.74, 20.65, 20.38. ESI-MS m/z calcd. for C18H20O6N3F [M + H]+ 394.1. Found: 394.1. HRMS for C18H20O6N3F [M + H]+ 394.1414. Found: 394.1414.
Furan glucosyl-1,2,3-triazole (5-e). Yield: 84.7%. Oily.1H-NMR (CDCl3): δ 8.27–7.26 (m, 5H, ArH, CCHN), 6.45–6.15 (m, 1H, H-1, α and β), 5.28–3.92 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.53–3.47 (m, 3H, OMe, α and β), 2.15–2.03 (m, 6H, Me2-CO, α and β); 13C-NMR (CDCl3): δ 169.61, 169.24, 147.46, 145.81, 136.94, 126.29, 124.38, 122.70, 122.39, 99.82, 93.95, 82.86, 82.31, 81.71, 78.55, 78.00, 76.20, 58.48, 58.33, 50.86, 50.32, 21.26, 20.89, 20.79, 20.53. ESI-MS m/z calcd. for C18H21O8N4 [M + H]+ 421.1. Found: 421.1. HRMS for C18H21O8N4 [M + H]+ 421.1359. Found: 421.1357.
Furan glucosyl-1,2,3-triazole (5-f). Yield: 82.9%. White solid, m.p. 131.3–133.0 °C. 1H-NMR (CDCl3): δ 7.87–7.27 (m, 5H, ArH, CCHN), 6.45–6.15 (m, 1H, H-1, α and β), 5.27–3.90 (m, 5H, H-2, H-3, H-4, H-5, H-6, α and β), 3.52–3.46 (m, 3H, OMe, α and β), 2.15–2.04 (m, 6H, Me2-CO, α and β); 13C-NMR (CDCl3): δ 169.61, 169.56, 169.25, 146.95, 134.02, 129.21, 129.14, 127.12, 121.21, 120.92, 99.82, 94.05, 82.81, 82.40, 81.81, 78.59, 78.21, 76.18, 58.47, 58.29, 50.53, 50.03, 21.28, 20.92, 20.82, 20.55. ESI-MS m/z calcd. for C18H21O6N3Cl [M + H]+ 410.1. Found: 410.1. HRMS for C18H21O6N3Cl [M + H]+ 410.1119. Found: 410.1120.
Furan glucosyl-1,2,3-triazole (F-a). Yield: 90.3%. White solid, m.p. 148.0–149.3 °C. 1H-NMR (CDCl3): δ 7.70–7.21 (m, 11H, ArH, CCHN), 6.40–6.10 (m, 1H, H-1, α and β), 5.25–4.00 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β), 2.04–1.93 (m, 6H, Me2-CO); 13C-NMR (CDCl3): δ 169.50, 169.16, 147.71, 136.93, 136.73, 130.54, 128.77, 128.67, 128.61, 128.33, 128.24, 128.10, 128.01, 127.98, 125.72, 121.16, 120.89, 99.70, 94.03, 81.67, 80.12, 80.05, 78.92, 78.12, 76.48, 72.43, 72.18, 50.35, 49.86, 21.13, 20.78, 20.69, 20.42. ESI-MS m/z calcd. for C24H26O6N3 [M + H]+ 452.1. Found: 452.1. HRMS for C24H26O6N3 [M + H]+ 452.1822. Found: 452.1821.
Furan glucosyl-1,2,3-triazole (6-b). Yield: 91.6%. White solid, m.p. 110.7–112.5 °C. 1H-NMR (CDCl3): δ 7.67–6.95 (m, 10H, ArH, CCHN), 6.35–6.06 (m, 1H, H-1, α and β), 5.20–3.96 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β) 2.22 (s, 3H, Ar-CH3), 1.97–1.86 (m, 6H, Me2-CO, α and β); 13C-NMR (CDCl3): δ 169.25, 168.90, 147.43, 138.12, 136.84, 136.65, 130.28, 128.58, 128.44, 128.38, 128.33, 128.00, 127.92, 127.75, 127.70, 126.08, 122.58, 120.98, 120.68, 99.40, 93.72, 81.43, 79.97, 79.79, 78.70, 76.84, 76.30, 72.16, 71.91, 50.09, 49.58, 21.13, 20.84, 20.49, 20.40, 20.14. ESI-MS m/z calcd. for C25H28O6N3 [M + H]+ 466.1. Found: 466.1. HRMS for C25H28O6N3 [M + H]+ 466.1978. Found: 466.1980.
Furan glucosyl-1,2,3-triazole (6-c). Yield: 92.5%. Yellow solid, m.p. 140.6–141.9 °C. 1H-NMR (CDCl3): δ 7.89–6.85 (m, 10H, ArH, CCHN), 6.49–6.19 (m, 1H, H-1, α and β), 5.24–4.03 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β), 3.83 (s, 3H, Ar-CH3), 2.11–2.04 (m, 6H, Me2-CO, α and β); 13C-NMR (CDCl3): δ 170.24, 169.87, 159.71, 159.66, 147.61, 147.55, 137.09, 137.06, 128.78, 128.72, 128.69, 128.31, 128.28, 128.06, 127.11, 123.51, 120.28, 120.12, 114.32, 114.29, 108.00, 100.77, 94.18, 80.80, 80.40, 80.34, 79.89, 78.62, 75.68, 72.51, 72.28, 55.40, 50.33, 49.90, 20.92, 20.81, 20.74, 20.54. ESI-MS m/z calcd. for C25H28O7N3 [M + H]+ 482.1. Found: 482.1. HRMS for C25H28O7N3 [M + H]+ 482.1927. Found: 482.1927.
Furan glucosyl-1,2,3-triazole (6-d). Yield: 87.1%. White solid, m.p. 123.8–124.9 °C. 1H-NMR (CDCl3): δ 7.68–6.98 (m, 10H, ArH, CCHN), 6.41–6.11 (m, 1H, H-1, α and β), 5.26–4.01 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β), 2.06–1.95 (m, 6H, Me2-CO); 13C-NMR (CDCl3): δ 169.66, 169.58, 169.49, 169.24, 163.93, 161.47, 146.96, 146.94, 136.94, 136.74, 128.75, 128.70, 128.43, 128.34, 128.08, 128.06, 127.57, 127.48, 120.97, 120.71, 115.91, 115.69, 99.77, 94.08, 81.74, 80.15, 80.08, 78.96, 78.16, 76.53, 72.51, 72.24, 50.51, 50.02, 21.20, 20.85, 20.77, 20.49. ESI-MS m/z calcd. for C24H25O6N3F [M + H]+ 470.1. Found: 470.1. HRMS for C24H25O6N3F [M + H]+: 470.1727. Found: 470.1736.
Furan glucosyl-1,2,3-triazole (6-e). Yield: 89.4%. Oily. 1H-NMR (CDCl3): δ 8.18–7.26 (m, 10H, ArH, CCHN), 6.42–6.12 (m, 1H, H-1, α and β), 5.28–4.06 (m, 7H, H-2, H-3, H-4, H-5, H-6, Ar-CH2, α and β) 2.07–1.96 (m, 6H, Me2-CO); 13C-NMR (CDCl3): δ 169.61, 169.25, 147.36, 145.63, 136.89, 136.86, 136.69, 128.79, 128.73, 128.49, 128.40, 128.09, 128.07, 126.22, 126.20, 124.28, 122.82, 122.53, 99.78, 93.98, 81.61, 80.21, 80.00, 78.94, 77.90, 76.54, 72.57, 72.28, 50.85, 50.34, 21.21, 20.86, 20.77, 20.49. ESI-MS m/z calcd. for C24H25O8N4 [M + H]+ 497.1. Found: 497.1. HRMS for C24H25O8N4 [M + H]+: 497.1672. Found: 497.1684.
Furan glucosyl-1,2,3-triazole (6-f). Yield: 92.8%. White solid, m.p. 140.6–141.4 °C. 1H-NMR (CDCl3): δ 7.78–7.31 (m, 10H, ArH, CCHN), 6.47–6.18 (m, 1H, H-1, α and β), 5.33–4.09 (m, 7H, H-2, H-3, H-4,H-5,H-6, Ar-CH2, α and β), 2.11–2.01 (m, 6H, Me2-CO); 13C-NMR (CDCl3): δ 169.49, 169.14, 149.62, 146.67, 136.91, 136.71, 136.11, 133.79, 129.15, 128.97, 128.70, 128.64, 128.37, 128.28, 128.02, 128.00, 126.98, 123.79, 121.25, 120.97, 99.71, 94.00, 81.65, 80.15, 80.03, 78.92, 78.04, 76.49, 72.47, 72.20, 50.48, 49.99, 21.14, 20.79, 20.70, 20.43. ESI-MS m/z calcd. for C24H25O6N3Cl [M + H]+ 486.1. Found: 486.1. HRMS for C24H25O6N3Cl [M + H]+ 486.1432. Found: 486.1437.