Effects of Lysozyme on the Activity of Ionic of Fluoroquinolone Species
Abstract
1. Introduction
2. Results and Discussion
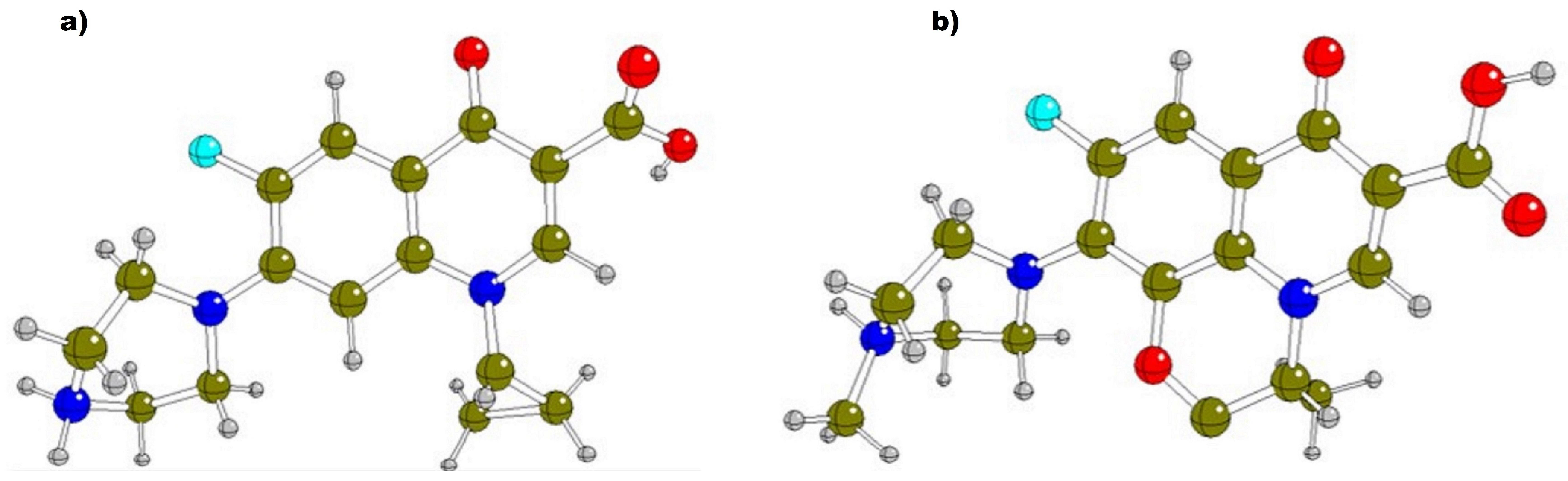
2.1. Optimization of FQs Species
2.2. Theoretical Stabilities and Reactivities of FQs in Aqueous Medium
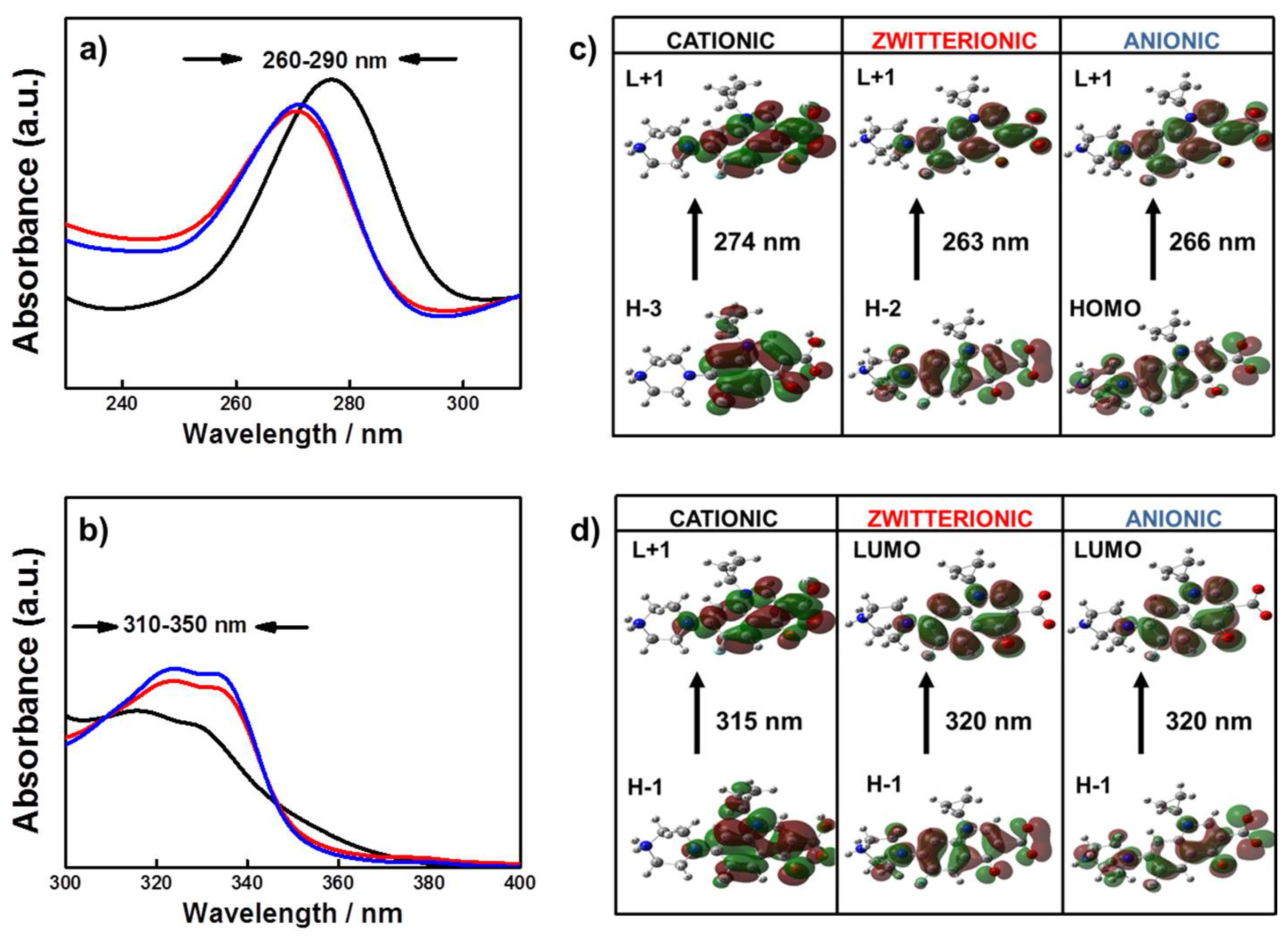
2.3. Spectral Differences of Anionic, Zwitterionic and Cationic FQs Species
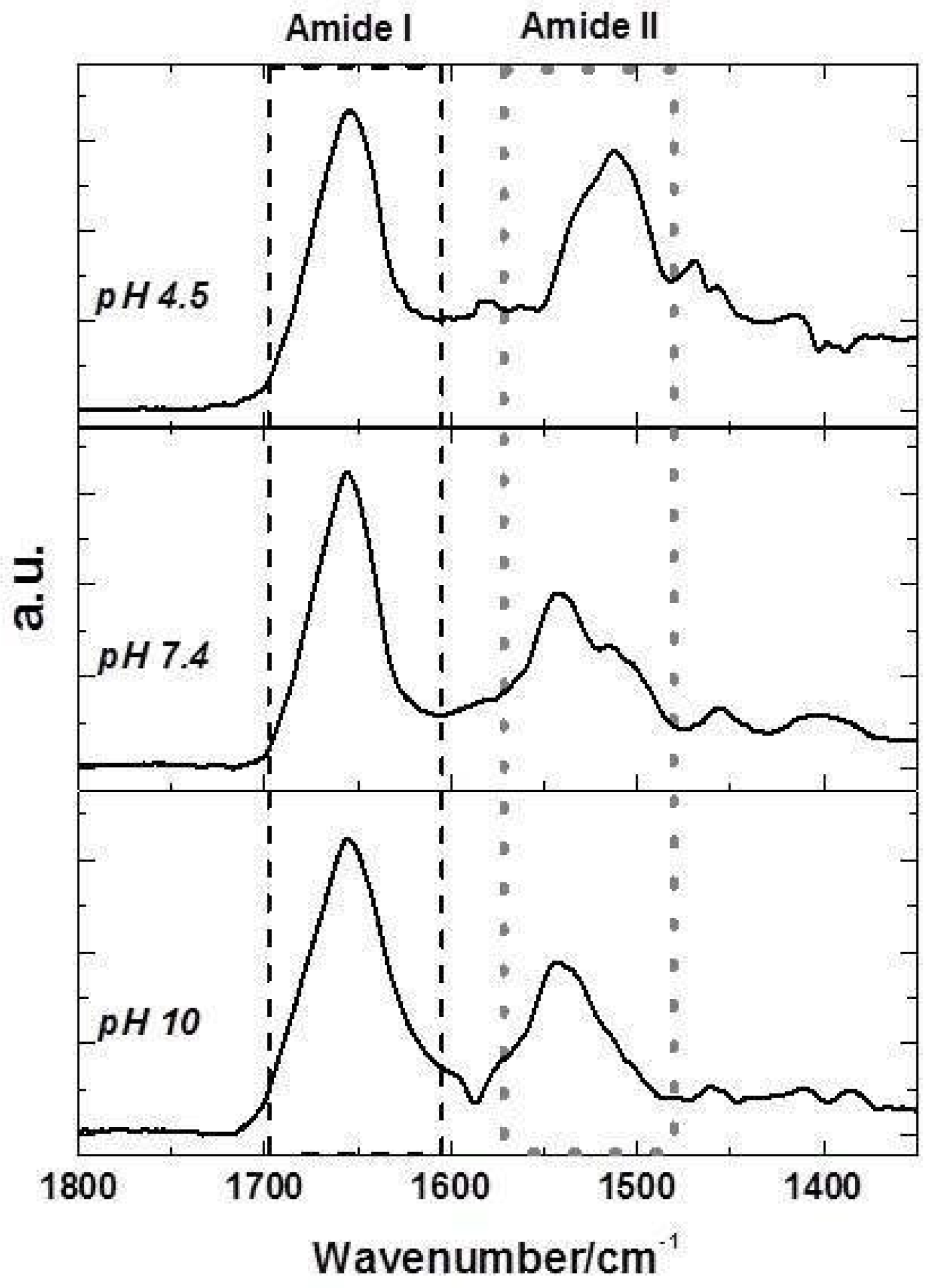
2.4. FTIR Spectra of FQs Species
2.5. Effects of FQs Molecules on the Structure of Lyz by FTIR Spectroscopy
2.6. Fluorescence Emission of FQs in Presence of Lyz
2.7. Thermodynamic Parameters and Binding Forces
2.8. Molecular Docking Studies of the FQs-Lyz Interactions
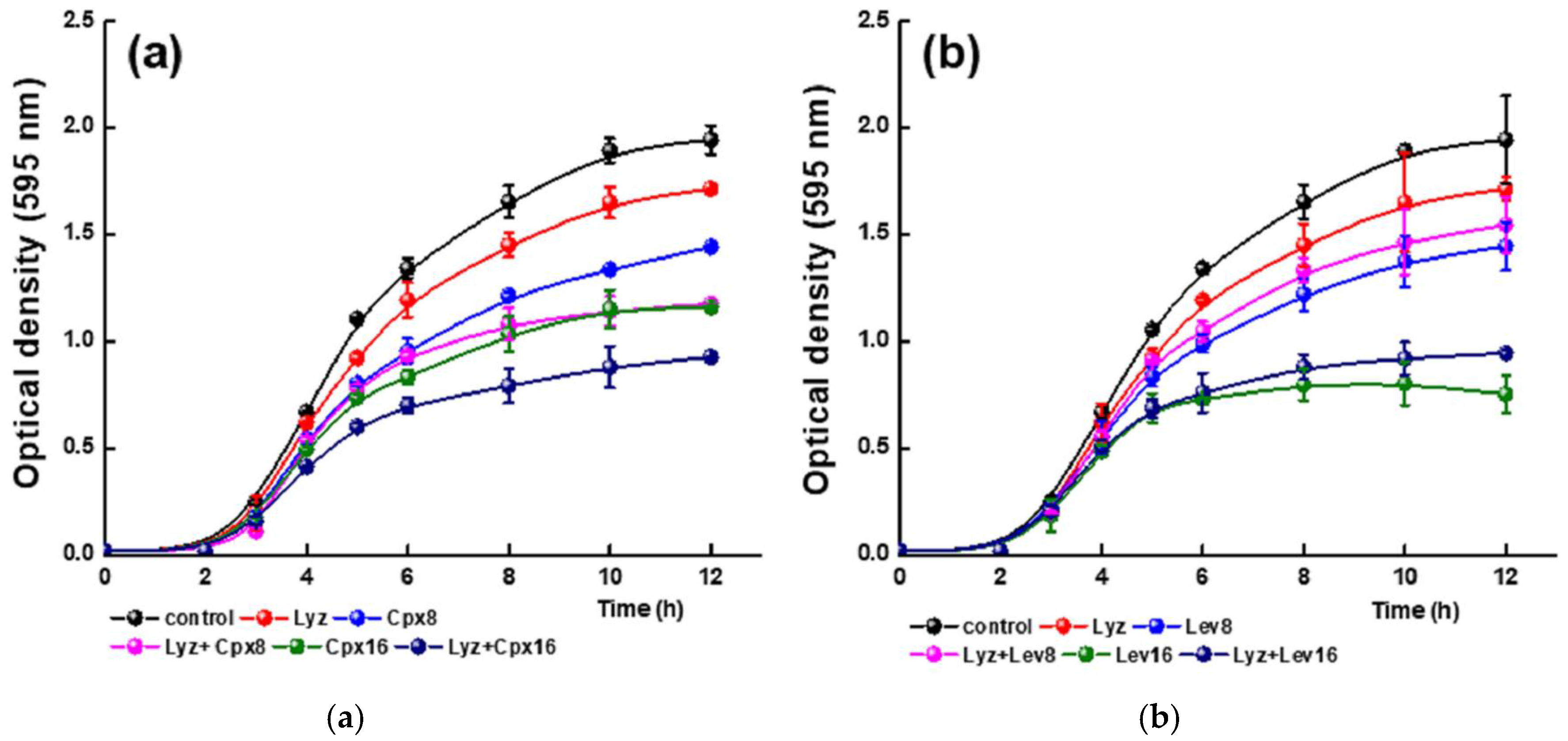
2.9. Effect of the Lyz-FQ Interactions on the Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Computational Calculations
3.3. UV-Visible Measurements
3.4. Fluorescence Assays at Different Temperatures
3.5. ATR-FTIR Measurements
3.6. Molecular Docking
3.7. Effect of Lyz on the Antimicrobial Activity of FQs
3.7.1. Microorganism and Culture Conditions
3.7.2. Viability Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Albini, A.; Monti, S. Photophysics and photochemistry of fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Jain, A.; Jain, S. Fluoroquinolone antibacterials: A review on chemistry, microbiology and therapeutic prospects. Acta Pol. Pharm. 2009, 66, 587–604. [Google Scholar] [PubMed]
- Gross, C.H.; Parsons, J.D.; Grossman, T.H.; Charifson, P.S.; Bellon, S.; Jernee, J.; Dwyer, M.; Chambers, S.P.; Markland, W.; Botfield, M. Active-site residues of Escherichia coli DNA gyrase required in coupling ATP hydrolysis to DNA supercoiling and amino acid substitutions leading to novobiocin resistance. Antimicrob. Agents Chemother. 2003, 47, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Fernandes, P.B. Structure-activity relationships of the fluoroquinolones. Antimicrob. Agents Chemother. 1989, 33, 131. [Google Scholar] [CrossRef] [PubMed]
- Khodursky, A.B.; Cozzarelli, N.R. The mechanism of inhibition of topoisomerase IV by quinolone antibacterials. J. Biol. Chem. 1998, 273, 27668–27677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The current case of quinolones: Synthetic approaches and antibacterial activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Neu, H. Quinolones as Broad-Spectrum Agents. In The 4-Quinolones: Anti Bacterial Agents In Vitro; Springer: London, UK, 1990; pp. 1–13. [Google Scholar]
- Vance-Bryan, K.; Guay, D.R.; Rotschafer, J.C. Clinical pharmacokinetics of ciprofloxacin. Clin. Pharmacokinet. 1990, 19, 434–461. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yano, I.; Masuda, S.; Hashimoto, Y.; Inui, K.-I. Distribution characteristics of levofloxacin and grepafloxacin in rat kidney. Pharm. Res. 1999, 16, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, M.L.; Guo, H.Y.; Wang, Y.C.; Wang, J.X. Synthesis and In Vitro Antibacterial Activity of 7-(3-Amino-6,7-dihydro-2-methyl-2H-pyrazolo[4,3-c]Pyridin-5(4H)-yl) Fluoroquinolone Derivatives. Molecules 2011, 16, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.C.; Allen, G.P.; Bearden, D.T. Levofloxacin in the treatment of complicated urinary tract infections and acute pyelonephritis. Ther. Clin. Risk Manag. 2008, 4843–4853. [Google Scholar] [CrossRef]
- Elisa, F.; Marianella, M.; Sandra, M.; Angelo, A. Unexpected Photoreactions of Some 7-Amino-6-fluoroquinolones in Phosphate Buffer. Eur. J. Org. Chem. 2001, 2001, 391–397. [Google Scholar]
- Lee, D.-S.; Han, H.-J.; Kim, K.; Park, W.-B.; Cho, J.-K.; Kim, J.-H. Dissociation and complexation of fluoroquinolone analogues. J. Pharm. Biomed. Anal. 1994, 12, 157–164. [Google Scholar] [CrossRef]
- Takács-Novák, K.; Noszál, B.; Hermecz, I.; Keresztúri, G.; Podányi, B.; Szász, G. Protonation equilibria of quinolone antibacterials. J. Pharm. Sci. 1990, 79, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulos, A.I.; Ioannou, P.C. Spectrofluorimetric study of the acid–base equilibria and complexation behavior of the fluoroquinolone antibiotics ofloxacin, norfloxacin, ciprofloxacin and pefloxacin in aqueous solution. Anal. Chim. Acta 1997, 354, 197–204. [Google Scholar] [CrossRef]
- Trivedi, P.; Vasudevan, D. Spectroscopic investigation of ciprofloxacin speciation at the goethite—Water interface. Environ. Sci. Technol. 2007, 41, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, H. Theoretical structure and vibrational spectra of ciprofloxacin: Density functional theory study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Rajalakshmi, K.; Kumaresan, S. Molecular Structure, Vibrational Spectroscopic Analysis of Levofloxacin by Density Functional method. J. Environ. Nanotechnol. 2013, 2, 6–9. [Google Scholar]
- Qin, P.; Su, B.; Liu, R. Probing the binding of two fluoroquinolones to lysozyme: A combined spectroscopic and docking study. Mol. Biosyst. 2012, 8, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Huang, P.L.; Kung, H.-f.; Blithe, D.L.; Chen, H.-C. Lysozyme and RNases as anti-HIV components in β-core preparations of human chorionic gonadotropin. Proc. Natl. Acad. Sci. USA 1999, 96, 2678–2681. [Google Scholar] [CrossRef] [PubMed]
- Chongqiu, J.; Li, L. Lysozyme enhanced europium–metacycline complex fluorescence: A new spectrofluorimetric method for the determination of lysozyme. Anal. Chim. Acta 2004, 511, 11–16. [Google Scholar] [CrossRef]
- Fang, Q.; Guo, C.; Wang, Y.; Liu, Y. The study on interactions between levofloxacin and model proteins by using multi-spectroscopic and molecular docking methods. J. Biomol. Struct. Dyn. 2017. [CrossRef] [PubMed]
- Pasban Ziyarat, F.; Asoodeh, A.; Sharif Barfeh, Z.; Pirouzi, M.; Chamani, J. Probing the interaction of lysozyme with ciprofloxacin in the presence of different-sized Ag nano-particles by multispectroscopic techniques and isothermal titration calorimetry. J. Biomol. Struct. Dyn. 2014, 32, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Ertem, M.Z. Calculating free energy changes in continuum solvation models. J. Phys. Chem. B 2016, 120, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, T.; Song, J.-W.; Suzuki, S.; Hirao, K. On Koopmans’ theorem in density functional theory. J. Chem. Phys. 2010, 133, 174101. [Google Scholar] [CrossRef] [PubMed]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Al-Mustafa, J. Magnesium, calcium, and barium perchlorate complexes of ciprofloxacin and norfloxacin. Acta Chim. Slov. 2002, 49, 457–466. [Google Scholar]
- Bilski, P.; Martinez, L.; Koker, E.; Chignell, C. Photosensitization by norfloxacin is a function of pH. Photochem. Photobiol. 1996, 64, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Pang, B.; Wang, T.; Zhao, T.; Yu, W. Investigation on the interactions of clenbuterol to bovine serum albumin and lysozyme by molecular fluorescence technique. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Hu, S.; Jing, C. Enrofloxacin sorption on smectite clays: Effects of pH, cations, and humic acid. J. Colloid Interface Sci. 2012, 372, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Kumar, R.T.; Ponnusamy, S. Vibrational spectra and normal coordinate analysis of diazepam, phenytoin and phenobarbitone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Anita, B. Spectral investigation and normal coordinate analysis of piperazine. Indian J. Pure Appl. Phys. 2008, 46, 833–838. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.A.; Ruysschaert, J.; Goormaghtigh, E. The optimization of protein secondary structure determination with infrared and circular dichroism spectra. Eur. J. Biochem. 2004, 271, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Wei, D.; Jiang, X.; Liu, Y.; Li, Z.; Li, N.; Li, K.; Liu, F.; Lai, L. Resurveying the Tris buffer solution: The specific interaction between tris (hydroxymethyl) aminomethane and lysozyme. Anal. Biochem. 2008, 378, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Griebenow, K. Fourier-transform infrared spectroscopic investigation of the thermal denaturation of hen egg-white lysozyme dissolved in aqueous buffer and glycerol. Biotechnol. Lett. 2000, 22, 1899–1905. [Google Scholar] [CrossRef]
- Das, I.; Halder, M. Counterpointing Scenarios on the Fate of Different Prototropic Forms of Norfloxacin Housed in the Pocket of Lysozyme: The Nonelectrostatic Interactions in the Protein Interior Are in the Controlling Role on the Prototropic Equilibria of the Guest. ACS Omega 2017, 2, 5504–5517. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Chi, Z.; Liu, R.; Teng, Y.; Fang, X.; Gao, C. Binding of oxytetracycline to bovine serum albumin: Spectroscopic and molecular modeling investigations. J. Agric. Food Chem. 2010, 58, 10262–10269. [Google Scholar] [CrossRef] [PubMed]
- Paramaguru, G.; Kathiravan, A.; Selvaraj, S.; Venuvanalingam, P.; Renganathan, R. Interaction of anthraquinone dyes with lysozyme: Evidences from spectroscopic and docking studies. J. Hazard. Mater. 2010, 175, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Sarwar, S.; Chakrabarti, P. The effect of the binding of ZnO nanoparticle on the structure and stability of α-lactalbumin: A comparative study. J. Phys. Chem. B 2013, 117, 13397–13408. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; Font, G.M.; Raya, R.R.; de Almeida, A.M.; Fadda, S.G. Proteomic analysis of the probiotic Lactobacillus reuteri CRL 1098 reveals novel tolerance biomarkers to bile acid-induced stress. Food Res. Int. 2015, 77, 599–607. [Google Scholar] [CrossRef]
- Patricelli, P.; Dell’elce, A.; Weidmann, C.; Ramirez, E.; Rossa, C.P.; Aguirre, M.S.; Cadoche, L.; Formentini, E. Actividad antibacteriana in vitro de Ciprofloxacina sobre una cepa autóctona de Escherichia coli: Efecto del pH sobre su potencia y efecto de la persistencia bacteriana sobre su modo de acción. FAVE Secc. Cienc. Vet. 2012, 15, 38–47. [Google Scholar] [CrossRef]
- Li, H.; Bu, X.; Lu, J.; Xu, C.; Wang, X.; Yang, X. Interaction study of ciprofloxacin with human telomeric DNA by spectroscopy and molecular docking. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Frisch, A.; Dennington, R.; Keith, T.; Millam, J.; Nielsen, A.; Holder, A.; Hiscocks, J. Gauss View Manual Version 5.0; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhang, G.; Zhang, L.; Yang, D.; Zhang, N.; He, L.; Du, G.; Lu, Y. Salt screening and characterization of ciprofloxacin. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Sukumar, N.; Vijayan, M. Hydration, mobility and accessibility of lysozyme: Structures of a pH 6.5 orthorhombic form and its low-humidity variant and a comparative study involving 20 crystallographically independent molecules. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 1110–1119. [Google Scholar] [CrossRef]
- Sukumar, N.; Biswal, B.; Vijayan, M. Structures of orthorhombic lysozyme grown at basic pH and its low-humidity variant. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999, 55, 934–937. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| Ciprofloxacin | ||||
|---|---|---|---|---|
| pH | E | ΔE (kJ/mol) | ΔGsolv (kJ/mol) | μ(D) |
| 4.5 | −1148.74 | 0 | −385.9 | 42.2 |
| 7.4 | −1148.28 | 1206 | −440.7 | 61.9 |
| 10 | −1147.81 | 2413 | −348.9 | 36.2 |
| Levofloxacin | ||||
| 4.5 | −1261.97 | 0 | −426.9 | 31.7 |
| 7.4 | −1261.51 | 263.4 | −515.5 | 58.6 |
| 10 | −1261.04 | 558.2 | −418.7 | 33.9 |
| LYSOZYME | |||||||
|---|---|---|---|---|---|---|---|
| 1606 cm−1 | 1623 cm−1 | 1630 cm−1 | 1640 cm−1 | 1652 cm−1 | 1670 cm−1 | 1690 cm−1 | |
| pH | Extended | Parallel β-Sheet | Antiparallel β-Sheet | Unordered Coil | α-Helix | Turn | Parallel β-Sheet |
| 4.5 | 15 ± 3 | 12 ± 1 | 24 ± 4 | 29 ± 1 | 20 ± 1 | 10 ± 1 | |
| 7.4 | 10 ± 2 | 5 ± 1 | 30 ± 2 | 31 ± 3 | 24 ± 1 | ||
| 10 | 8 ± 1 | 9 ± 2 | 11 ± 2 | 23 ± 3 | 21 ± 2 | 18 ± 1 | |
| LYSOZYME—CIPROFLOXACIN | |||||||
| 4.5 | 15 ± 1 | 17 ± 2 | 22 ± 1 | 28 ± 2 | 18 ± 1 | ||
| 7.4 | 18 ± 1 | 14 ± 1 | 20 ± 3 | 29 ± 2 | 19 ± 3 | ||
| 10 | 10 ± 3 | 6 ± 1 | 11 ± 2 | 20 ± 1 | 27 ± 1 | 18 ± 1 | 8 ± 1 |
| LYSOZYME—LEVOFLOXACIN | |||||||
| 4.5 | 21 ± 2 | 17 ± 1 | 16 ± 3 | 29 ± 2 | 17 ± 1 | ||
| 7.4 | 18 ± 2 | 18 ± 3 | 19 ± 3 | 26 ± 1 | 19 ± 1 | ||
| 10 | 18 ± 1 | 10 ± 1 | 7 ± 2 | 18 ± 2 | 25 ± 2 | 14 ± 2 | 8 ± 2 |
| pH = 4.5 | pH = 7.4 | pH = 10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K (104 L mol−1) | SD * | r ** | K (104 L mol−1) | SD * | r ** | K (104 L mol−1) | SD * | r ** | ||
| Lyz—Cpx | 298 K | 0.41 | 0.01 | 0.997 | 1.80 | 0.01 | 0.988 | 4.05 | 0.01 | 0.994 |
| 308 K | 0.31 | 0.02 | 0.983 | 1.13 | 0.02 | 0.992 | 3.24 | 0.02 | 0.995 | |
| Lyz—Lev | 298 K | 0.97 | 0.02 | 0.988 | 0.27 | 0.02 | 0.991 | 15.15 | 0.02 | 0.994 |
| 308 K | 0.69 | 0.10 | 0.982 | 0.22 | 0.10 | 0.989 | 13.93 | 0.10 | 0.987 | |
| pH | ΔH (kJ mol−1) | ΔS (J mol−1K−1) | ΔG (kJ mol−1) | r | |
|---|---|---|---|---|---|
| Cpx-Lyz | 4.5 | −24.04 ± 0.90 | −11.32 ± 2.93 | −20.64 ± 0.10 | 0.997 |
| 7.4 | −37.55 ± 1.73 | −44.46 ± 5.78 | −24.27 ± 0.22 | 0.996 | |
| 10 | −15.53 ± 1.26 | 35.95 ± 4.21 | −26.28 ± 0.26 | 0.987 | |
| Lev-Lyz | 4.5 | −26.27 ± 1.14 | −11.82 ± 1.12 | −22.76 ± 0.07 | 0.989 |
| 7.4 | −14.98 ± 0.84 | 15.32 ± 2.45 | −19.57 ± 0.15 | 0.993 | |
| 10 | −6.39 ± 1.22 | 77.70 ± 4.13 | −29.55 ± 0.31 | 0.998 |
| Inhibition of Growth (%) | ||
|---|---|---|
| Treatments | pH = 4.5 | pH = 7.5 |
| Lyz 2 mg/mL | 8.65 ± 0.7 a | 18.70 ± 1.1 a |
| Cpx 8 μg/mL | 29.96 ± 1.5 b | 25.84 ± 1.7 b |
| Cpx 8 μg/mL + Lyz | 14.58 ± 0.9 c | 39.57 ± 1.3 c |
| Cpx 16 μg/mL | 42.83 ± 2.2 d | 40.33 ± 2.3 c |
| Cpx 16 μg/mL + Lyz | 30.22 ± 1.6 b | 52.32 ± 3.3 d |
| Lev 8 μg/mL | 15.99 ± 0.9 c | 25.69 ± 1.5 b |
| Lev 8 μg/mL + Lyz | 26.54 ± 2.5 e | 20.50 ± 2.2 d |
| Lev 16 μg/mL | 18.93 ± 1.7 f | 61.30 ± 5.8 e |
| Lev 16 μg/mL + Lyz | 29.36 ± 3.5 b | 51.40 ± 4.8 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, H.A.; Bustos, A.Y.; Taranto, M.P.; Frías, M.D.l.A.; Ledesma, A.E. Effects of Lysozyme on the Activity of Ionic of Fluoroquinolone Species. Molecules 2018, 23, 741. https://doi.org/10.3390/molecules23040741
Perez HA, Bustos AY, Taranto MP, Frías MDlA, Ledesma AE. Effects of Lysozyme on the Activity of Ionic of Fluoroquinolone Species. Molecules. 2018; 23(4):741. https://doi.org/10.3390/molecules23040741
Chicago/Turabian StylePerez, Hugo Alejandro, Ana Yanina Bustos, María Pía Taranto, María De los Angeles Frías, and Ana Estela Ledesma. 2018. "Effects of Lysozyme on the Activity of Ionic of Fluoroquinolone Species" Molecules 23, no. 4: 741. https://doi.org/10.3390/molecules23040741
APA StylePerez, H. A., Bustos, A. Y., Taranto, M. P., Frías, M. D. l. A., & Ledesma, A. E. (2018). Effects of Lysozyme on the Activity of Ionic of Fluoroquinolone Species. Molecules, 23(4), 741. https://doi.org/10.3390/molecules23040741








