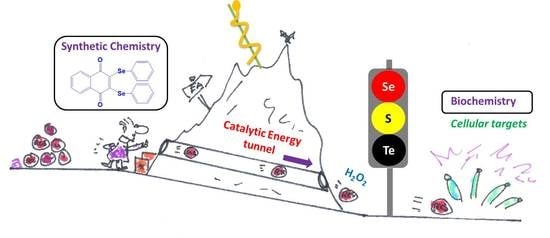
Small Molecule Catalysts with Therapeutic Potential
Abstract
:1. Introduction
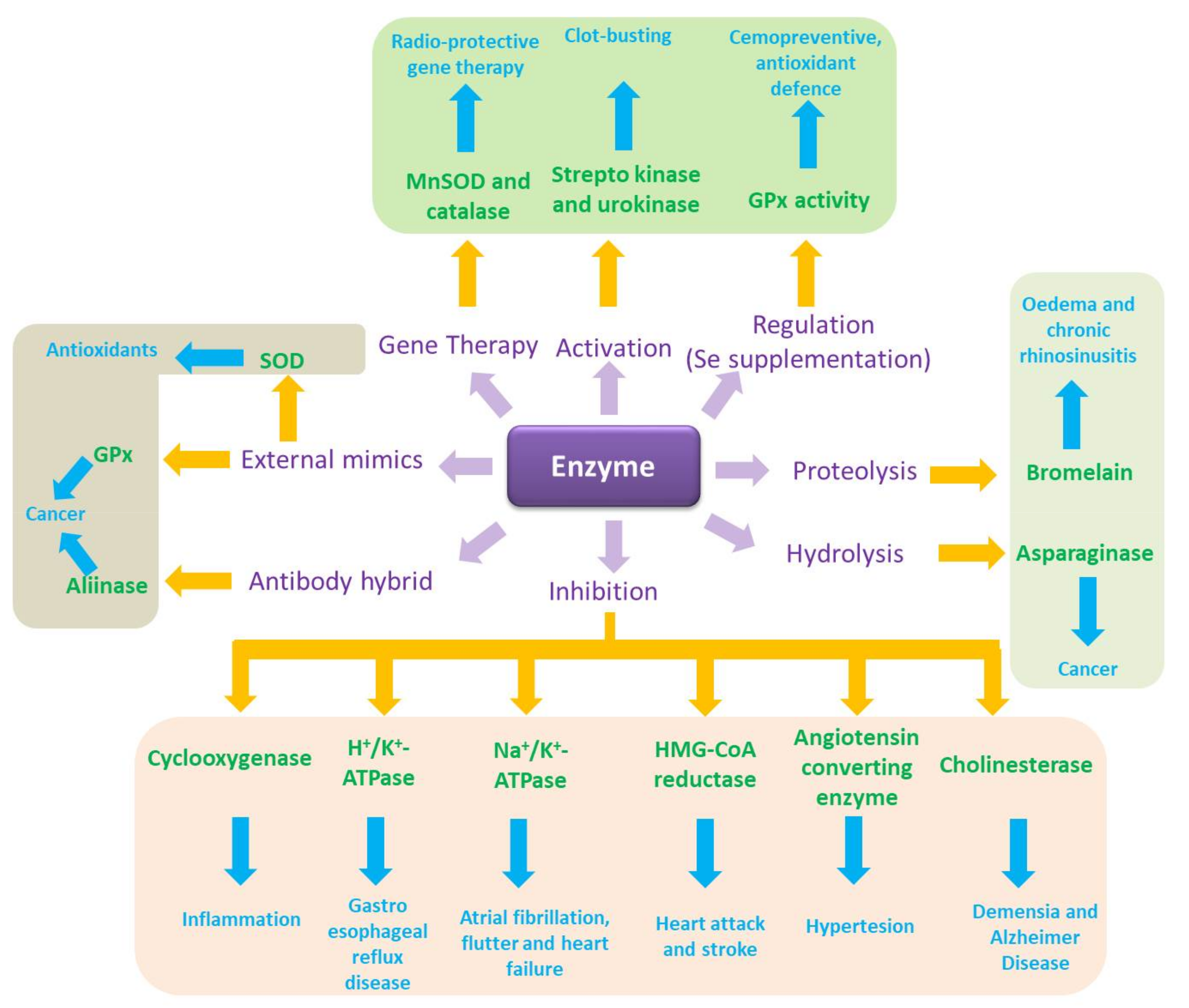
2. The Use of Enzymes in Therapy
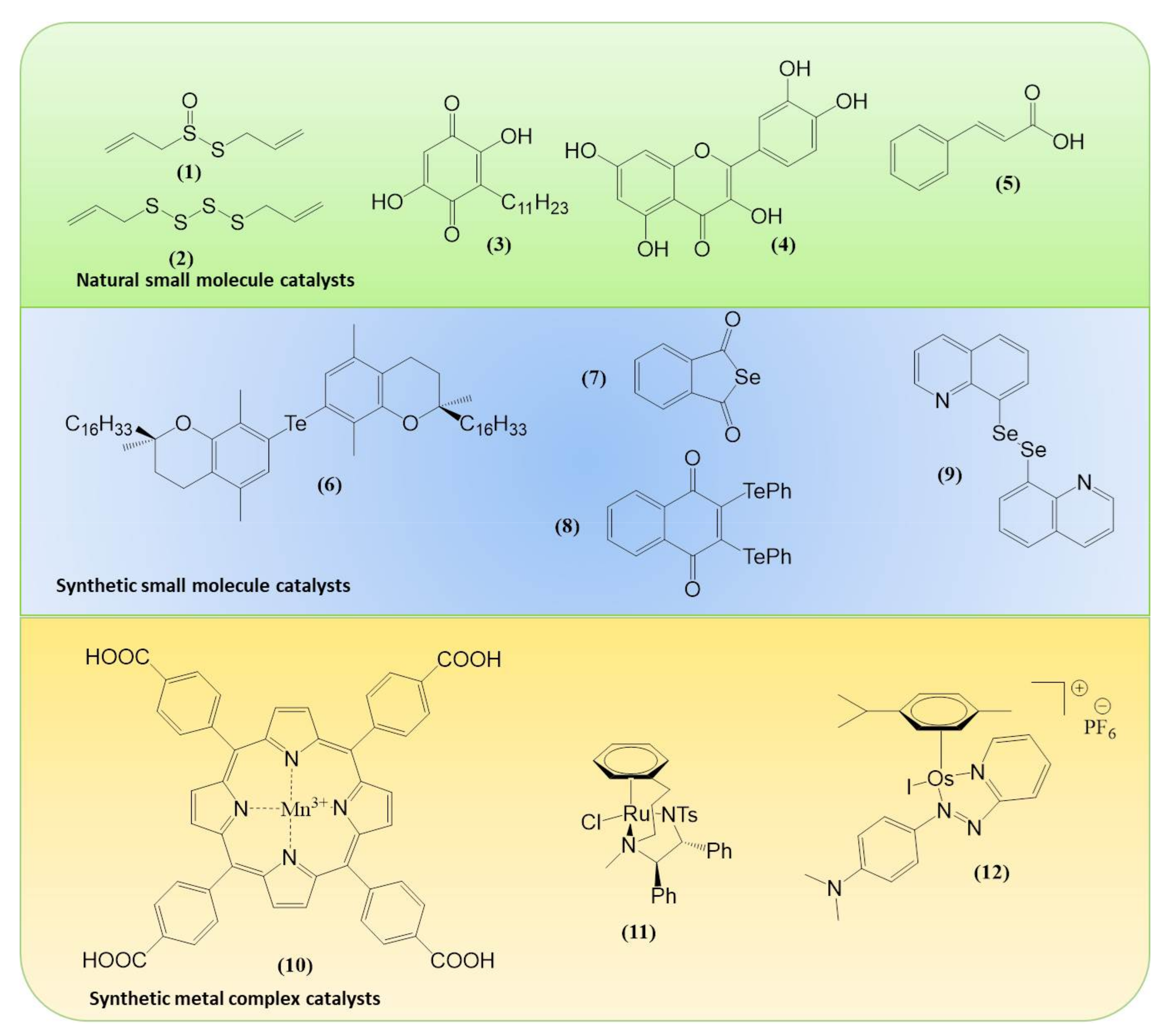
3. From Enzyme Mimics to Artificial Catalysts
4. Redox Catalysts with Sensor/Effector Properties
5. Targets for Redox Catalysts: A Selected Many
6. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cuba: Civil Society a ‘Catalyst of Change,’ Says Obama. Available online: https://www.demdigest.org/cuba-civil-society-catalyst-change-says-obama/ (accessed on 2 February 2018).
- Hanefeld, U.; Lefferts, L. Catalysis: An Integrated Textbook for Students; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Ahmad, S.; Muthukumar, S.; Kuncha, S.K.; Routh, S.B.; Yerabham, A.S.; Hussain, T.; Kamarthapu, V.; Kruparani, S.P.; Sankaranarayanan, R. Specificity and catalysis hardwired at the RNA-protein interface in a translational proofreading enzyme. Nat. Commun. 2015, 6, 7552. [Google Scholar] [CrossRef] [PubMed]
- Barbas, A.; Matos, R.G.; Amblar, M.; Lopez-Vinas, E.; Gomez-Puertas, P.; Arraiano, C.M. Determination of key residues for catalysis and RNA cleavage specificity: One mutation turns RNase II into a “super-enzyme”. J. Biol. Chem. 2009, 284, 20486–20498. [Google Scholar] [CrossRef] [PubMed]
- Cong, P.; Shuman, S. Covalent catalysis in nucleotidyl transfer. A KTDG motif essential for enzyme-GMP complex formation by mRNA capping enzyme is conserved at the active sites of RNA and DNA ligases. J. Biol. Chem. 1993, 268, 7256–7260. [Google Scholar] [PubMed]
- Hertel, K.J.; Peracchi, A.; Uhlenbeck, O.C.; Herschlag, D. Use of intrinsic binding energy for catalysis by an RNA enzyme. Proc. Natl. Acad. Sci. USA 1997, 94, 8497–8502. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Dey, M.; Neculai, D.; Cao, C.; Dever, T.E.; Sicheri, F. Structure of the dual enzyme ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 2008, 132, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.H.; Liu, Z.; Brennan, J.D.; Li, Y. An efficient RNA-cleaving DNA enzyme that synchronizes catalysis with fluorescence signaling. J. Am. Chem. Soc. 2003, 125, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Minajigi, A.; Francklyn, C.S. RNA-assisted catalysis in a protein enzyme: The 2′-hydroxyl of tRNA(Thr) A76 promotes aminoacylation by threonyl-tRNA synthetase. Proc. Natl. Acad. Sci. USA 2008, 105, 17748–17753. [Google Scholar] [CrossRef] [PubMed]
- Narlikar, G.J.; Gopalakrishnan, V.; McConnell, T.S.; Usman, N.; Herschlag, D. Use of binding energy by an RNA enzyme for catalysis by positioning and substrate destabilization. Proc. Natl. Acad. Sci. USA 1995, 92, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Shuman, S.; Liu, Y.; Schwer, B. Covalent catalysis in nucleotidyl transfer reactions: Essential motifs in Saccharomyces cerevisiae RNA capping enzyme are conserved in Schizosaccharomyces pombe and viral capping enzymes and among polynucleotide ligases. Proc. Natl. Acad. Sci. USA 1994, 91, 12046–12050. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; The Central Role of Enzymes as Biological Catalysts; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Frey, P.A. Radical mechanisms of enzymatic catalysis. Annu. Rev. Biochem. 2001, 70, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hesek, D.; Suvorov, M.; Lee, W.; Vakulenko, S.; Mobashery, S. A mechanism-based inhibitor targeting the dd-transpeptidase activity of bacterial penicillin-binding proteins. J. Am. Chem. Soc. 2003, 125, 16322–16326. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.L.; Rower, J.E. Zidovudine and lamivudine for HIV infection. Clin. Med. Rev. Ther. 2010, 2. [Google Scholar] [CrossRef]
- Brown, P.M.; Pratt, A.G.; Isaacs, J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N. The mechanism of action of methotrexate. Rheum. Dis. Clin. 1997, 23, 739–755. [Google Scholar] [CrossRef]
- Schiavone, S.; Trabace, L. Small molecules: Therapeutic application in neuropsychiatric and neurodegenerative disorders. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Ganiats, T.G.; Norcross, W.A.; Halverson, A.L.; Burford, P.A.; Palinkas, L.A. Does beano prevent gas—A double-blind crossover study of oral alpha-galactosidase to treat dietary oligosaccharide intolerance. J. Fam. Pract. 1994, 39, 441–445. [Google Scholar] [PubMed]
- Lettieri, J.T.; Dain, B. Effects of beano on the tolerability and pharmacodynamics of acarbose. Clin. Ther. 1998, 20, 497–504. [Google Scholar] [CrossRef]
- Fieker, A.; Philpott, J.; Armand, M. Enzyme replacement therapy for pancreatic insufficiency: Present and future. Clin. Exp. Gastroenterol. 2011, 4, 55–73. [Google Scholar] [PubMed]
- Ouriel, K.; Welch, E.L.; Shortell, C.K.; Geary, K.; Fiore, W.M.; Cimino, C. Comparison of streptokinase, urokinase, and recombinant tissue-plasminogen activator in an in vitro model of venous thrombolysis. J. Vasc. Surg. 1995, 22, 593–597. [Google Scholar] [CrossRef]
- Egler, R.A.; Ahuja, S.P.; Matloub, Y. l-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J. Pharmacol. Pharmacother. 2016, 7, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Avramis, V.I.; Tiwari, P.N. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int. J. Nanomed. 2006, 1, 241–254. [Google Scholar]
- Cachumba, J.J.M.; Antunes, F.A.F.; Peres, G.F.D.; Brumano, L.P.; Dos Santos, J.C.; Da Silva, S.S. Current applications and different approaches for microbial l-asparaginase production. Braz. J. Microbiol. 2016, 47, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Thomas, X.; Bertrand, Y.; Baruchel, A.; Blin, N.; Tavernier, E.; Ducassou, S.; Vey, N.; Gandemer, V.; Cacheux, V.; Mazingue, F.; et al. Pharmacokinetic and pharmacodynamic characterization of graspa versus native l-asparaginase in combination with cooprall chemotherapy in a phase 3 randomized trial for the treatment of patients with relapsed acute lymphoblastic leukemia (NCT01 51851 7). Blood 2015, 126, 2492. [Google Scholar]
- Angiolillo, A.L.; Schore, R.J.; Devidas, M.; Borowitz, M.J.; Carroll, A.J.; Gastier-Foster, J.M.; Heerema, N.A.; Keilani, T.; Lane, A.R.; Loh, M.L.; et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli l-asparaginase in the treatment of patients with acute lymphoblastic leukemia: Results from Children’s Oncology Group Study AALL07P4. J. Clin. Oncol. 2014, 32, 3874–3882. [Google Scholar] [CrossRef] [PubMed]
- Luniak, N.; Meiser, P.; Burkart, S.; Muller, R. Heterologous expression of the plant cysteine protease bromelain and its inhibitor in Pichia pastoris. Biotechnol. Prog. 2017, 33, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Buttner, L.; Achilles, N.; Bohm, M.; Shah-Hosseini, K.; Mosges, R. Efficacy and tolerability of bromelain in patients with chronic rhinosinusitis—A pilot study. B-ENT 2013, 9, 217–225. [Google Scholar] [PubMed]
- Xu, Z.J.; Du, P.; Meiser, P.; Jacob, C. Proanthocyanidins: Oligomeric structures with unique biochemical properties and great therapeutic promise. Nat. Prod. Commun. 2012, 7, 381–388. [Google Scholar] [PubMed]
- Sakata, V.M.; da Silva, F.T.; Hirata, C.E.; Marin, M.L.C.; Rodrigues, H.; Kalil, J.; Costa, R.A.; Yamamoto, J.H. High rate of clinical recurrence in patients with Vogt-Koyanagi-Harada disease treated with early high-dose corticosteroids. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Carpenter, M.; Agarwal, A.; Mitra, P.; Nie, S.; Greenberger, J.S. Intraoral manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo 2004, 18, 401–410. [Google Scholar] [PubMed]
- Hartmann, J. 2017 clinical trials update: Innovations in hemophilia therapy. Am. J. Hematol. 2016, 91, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Stolberg, S.G. The biotech death of Jesse Gelsinger. The New York Times Magazine, 28 November 1999. [Google Scholar]
- Nathwani, A.C.; Reiss, U.M.; Tuddenham, E.G.D.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014, 371, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.S.; Chew, A.J.; Hutchison, S.; Larson, P.J.; Herzog, R.W.; Arruda, V.P.; Tai, S.J.; Ragni, M.V.; Thompson, A.; Ozelo, M.; et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003, 101, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.S.; Arruda, V.R.; Pierce, G.F.; Glader, B.; Ragni, M.; Rasko, J.; Ozelo, M.C.; Hoots, K.; Blatt, P.; Konkle, B.; et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006, 12, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Liew, A.T.F.; Theis, T.; Jensen, S.O.; Garcia-Lara, J.; Foster, S.J.; Firth, N.; Lewis, P.J.; Harry, E.J. A simple plasmid-based system that allows rapid generation of tightly controlled gene expression in Staphylococcus aureus. Microbiology 2011, 157, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Leese, M.P.; Mouzakiti, A.; Purohit, A.; Potter, B.V.L.; Reed, M.J.; Packham, G. 2-MeOE2bisMATE induces caspase-dependent apoptosis in CAL51 breast cancer cells and overcomes resistance to TRAIL via cooperative activation of caspases. Apoptosis 2004, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Leese, M.P.; Leblond, B.; Woo, L.W.L.; Ganeshapillai, D.; Purohit, A.; Reed, M.J.; Potter, B.V.L.; Packham, G. Inhibition of superoxide dismutase by 2-methoxyoestradiol analogues and oestrogen derivatives: Structure-activity relationships. Anti-Cancer Drug Des. 2001, 16, 209–215. [Google Scholar]
- Venardos, K.; Harrison, G.; Headrick, J.; Perkins, A. Effects of dietary selenium on glutathione peroxidase and thioredoxin reductase activity and recovery from cardiac ischemia-reperfusion. J. Trace Elem. Med. Biol. 2004, 18, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Mironchik, M.; Mirelman, D.; Wilchek, M.; Rabinkov, A. Inhibition of tumor growth by a novel approach: In situ allicin generation using targeted alliinase delivery. Mol. Cancer Ther. 2003, 2, 1295–1301. [Google Scholar] [PubMed]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M. Site-Specific in Situ Generation of Allicin Using a Targeted Alliinase Delivery System for the Treatment of Cancers, Tumors, Infectious Diseases and Other Allicin-Sensitive Diseases. U.S. Patent US7445802B2, 4 November 2008. [Google Scholar]
- Fry, F.H.; Okarter, N.; Baynton-Smith, C.; Kershaw, M.J.; Talbot, N.J.; Jacob, C. Use of a substrate/alliinase combination to generate antifungal activity in situ. J. Agric. Food Chem. 2005, 53, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Dosz, E.B.; Sun, J.; Hoeflinger, J.L.; Van Tassell, M.L.; Chen, P.; Harnly, J.M.; Miller, M.J.; Jeffery, E.H. Myrosinase-dependent and -independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front. Plant. Sci. 2015, 6, 831. [Google Scholar] [CrossRef] [PubMed]
- Estevam, E.C.; Griffin, S.; Nasim, M.J.; Zielinski, D.; Aszyk, J.; Osowicka, M.; Dawidowska, N.; Idroes, R.; Bartoszek, A.; Jacob, C. Inspired by nature: The use of plant-derived substrate/enzyme combinations to generate antimicrobial activity in situ. Nat. Prod. Commun. 2015, 10, 1733–1738. [Google Scholar] [PubMed]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Cipolla Colt. Available online: https://en.wikipedia.org/wiki/Cry,_Onion! (accessed on 2 February 2018).
- Walters, W.P. Going further than lipinski’s rule in drug design. Expert Opin. Drug Dis. 2012, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Babu, R. bImpeaching the President: Clinton and the Culture Clash, a review from India. Am. Stud. Int. 2001, 39, 69–75. [Google Scholar]
- Batinic-Haberle, I.; Reboucas, J.S.; Spasojevic, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed]
- Cline, J.M.; Dugan, G.; Bourland, J.D.; Perry, D.L.; Stitzel, J.D.; Weaver, A.A.; Jiang, C.; Tovmasyan, A.; Owzar, K.; Spasojevic, I.; et al. Post-irradiation treatment with a superoxide dismutase mimic, MnTnHex-2-PyP(5+), mitigates radiation injury in the lungs of non-human primates after whole-thorax exposure to ionizing radiation. Antioxidants 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Roy, P.; Karmodak, N.; Jemmis, E.D.; Mugesh, G. Nanoisozymes: Crystal-facet-dependent enzyme-mimetic activity of V2O5 nanomaterials. Angew. Chem. 2018, 57. [Google Scholar] [CrossRef]
- Witek, K.; Nasim, M.J.; Bischoff, M.; Gaupp, R.; Arsenyan, P.; Vasiljeva, J.; Marc, M.A.; Olejarz, A.; Latacz, G.; Kiec-Kononowicz, K.; et al. Selenazolinium salts as “small molecule catalysts” with high potency against ESKAPE bacterial pathogens. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Ebselen as an Add-On Treatment in Hypo/Mania. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03013400 (accessed on 2 February 2018).
- Jamier, V.; Ba, L.A.; Jacob, C. Selenium- and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chem. Eur. J. 2010, 16, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Saidu, N.E.B.; Intemann, J.; Jacob, C.; Montenarh, M. A new tellurium-containing amphiphilic molecule induces apoptosis in HCT116 colon cancer cells. Biochim. Biophys. Acta 2014, 1840, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Poon, J.F.; Engman, L. Catalytic antioxidants: Regenerable tellurium analogues of vitamin E. Org. Lett. 2013, 15, 6274–6277. [Google Scholar] [CrossRef] [PubMed]
- Poon, J.F.; Yan, J.J.; Singh, V.P.; Gates, P.J.; Engman, L. Regenerable radical-trapping tellurobistocopherol antioxidants. J. Org. Chem. 2016, 81, 12540–12544. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. Redox signalling via the cellular thiolstat. Biochem. Soc. Trans. 2011, 39, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.H.; Habtemariam, A.; Lopez-Clavijo, A.F.; Barrow, M.P.; Sadler, P.J.; O’Connor, P.B. Insights into the binding sites of organometallic ruthenium anticancer compounds on peptides using ultra-high resolution mass spectrometry. J. Am. Soc. Mass Spectrom. 2014, 25, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, J.P.C.; Romero-Canelón, I.; Sanchez-Cano, C.; Clarkson, G.J.; Habtemariam, A.; Wills, M.; Sadler, P.J. Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells. Nat. Chem. 2018, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ude, Z.; Romero-Canelon, I.; Twamley, B.; Hughes, D.F.; Sadler, P.J.; Marmion, C.J. A novel dual-functioning ruthenium(II)-arene complex of an anti-microbial ciprofloxacin derivative—Anti-proliferative and anti-microbial activity. J. Inorg. Biochem. 2016, 160, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sanchez-Cano, C.; Soni, R.; Romero-Canelon, I.; Hearn, J.M.; Liu, Z.; Wills, M.; Sadler, P.J. The contrasting catalytic efficiency and cancer cell antiproliferative activity of stereoselective organoruthenium transfer hydrogenation catalysts. Dalton Trans. 2016, 45, 8367–8378. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.M.; Romero-Canelon, I.; Munro, A.F.; Fu, Y.; Pizarro, A.M.; Garnett, M.J.; McDermott, U.; Carragher, N.O.; Sadler, P.J. Potent organo-osmium compound shifts metabolism in epithelial ovarian cancer cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3800–E3805. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Hui, S.; Ghergurovich, J.M.; Fan, J.; Intlekofer, A.M.; White, R.M.; Rabinowitz, J.D.; Thompson, C.B.; Zhang, J. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab. 2018, 27, 428–438.e5. [Google Scholar] [CrossRef] [PubMed]
- Fry, F.H.; Holme, A.L.; Giles, N.M.; Giles, G.I.; Collins, C.; Holt, K.; Pariagh, S.; Gelbrich, T.; Hursthouse, M.B.; Gutowski, N.J.; et al. Multifunctional redox catalysts as selective enhancers of oxidative stress. Org. Biomol. Chem. 2005, 3, 2579–2587. [Google Scholar] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- De Belleroche, J.; Orrell, R.; King, A. Familial amyotrophic lateral sclerosis/motor neurone disease (FALS): A review of current developments. J. Med. Genet. 1995, 32, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Yilmaz, R.; Muller, K.; Grehl, T.; Petri, S.; Meyer, T.; Grosskreutz, J.; Weydt, P.; Ruf, W.; Neuwirth, C.; et al. Hot-spot KIF5A mutations cause familial ALS. Brain 2018, 141, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxid. Med. Cell. Longev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Nicco, C.; Batteux, F. ROS modulator molecules with therapeutic potential in cancers treatments. Molecules 2017, 23. [Google Scholar] [CrossRef]
- Alexandre, J.; Nicco, C.; Chereau, C.; Laurent, A.; Weill, B.; Goldwasser, F.; Batteux, F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J. Natl. Cancer Inst. 2006, 98, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Heer, C.D.; Davis, A.B.; Riffe, D.B.; Wagner, B.A.; Falls, K.C.; Allen, B.G.; Buettner, G.R.; Beardsley, R.A.; Riley, D.P.; Spitz, D.R. Superoxide dismutase mimetic GC4419 enhances the oxidation of pharmacological ascorbate and its anticancer effects in an H2O2-dependent manner. Antioxidants 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.O.G.; Ignarro, L.J.; Lundstrom, I.; Jynge, P.; Almen, T. Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov. Today 2015, 20, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Coriat, R.; Alexandre, J.; Nicco, C.; Quinquis, L.; Benoit, E.; Chereau, C.; Lemarechal, H.; Mir, O.; Borderie, D.; Treluyer, J.M.; et al. Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir. J. Clin. Investig. 2014, 124, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Zhu, Y.X.; Tong, Q.; Kosmacek, E.A.; Lichter, E.Z.; Oberley-Deegan, R.E. The addition of manganese porphyrins during radiation inhibits prostate cancer growth and simultaneously protects normal prostate tissue from radiation damage. Antioxidants 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Giles, N.M.; Giles, G.I.; Holley, J.E.; Gutowski, N.J.; Jacob, C. Targeting oxidative stress-related diseases: Organochalcogen catalysts as redox sensitizers. Biochem. Pharmacol. 2003, 66, 2021–2028. [Google Scholar] [CrossRef]
- Collins, C.A.; Fry, F.H.; Holme, A.L.; Yiakouvaki, A.; Al-Qenaei, A.; Pourzand, C.; Jacob, C. Towards multifunctional antioxidants: Synthesis, electrochemistry, in vitro and cell culture evaluation of compounds with ligand/catalytic properties. Org. Biomol. Chem. 2005, 3, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Giles, N.M.; Collins, C.A.; Holt, K.; Fry, F.H.; Lowden, P.A.S.; Gutowski, N.J.; Jacob, C. Electrochemical, in vitro and cell culture analysis of integrated redox catalysts: Implications for cancer therapy. Chem. Commun. 2003, 2030–2031. [Google Scholar] [CrossRef]
- Doering, M.; Ba, L.A.; Lilienthal, N.; Nicco, C.; Scherer, C.; Abbas, M.; Zada, A.A.P.; Coriat, R.; Burkholz, T.; Wessjohann, L.; et al. Synthesis and selective anticancer activity of organochalcogen based redox catalysts. J. Med. Chem. 2010, 53, 6954–6963. [Google Scholar] [CrossRef] [PubMed]
- Manikova, D.; Letavayova, L.M.; Vlasakova, D.; Kosik, P.; Estevam, E.C.; Nasim, M.J.; Gruhlke, M.; Slusarenko, A.; Burkholz, T.; Jacob, C.; et al. Intracellular diagnostics: Hunting for the mode of action of redox-modulating selenium compounds in selected model systems. Molecules 2014, 19, 12258–12279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, P.; Viswanathan, U.M.; Khairan, K.; Buric, T.; Saidu, N.E.B.; Xu, Z.J.; Hanf, B.; Bazukyan, I.; Trchounian, A.; Hannemann, F.; et al. Synthesis of amphiphilic, chalcogen-based redox modulators with in vitro cytotoxic activity against cancer cells, macrophages and microbes. Med. Chem. Commun. 2014, 5, 25–31. [Google Scholar] [CrossRef]
- Lilienthal, N.; Prinz, C.; Peer-Zada, A.A.; Doering, M.; Ba, L.A.; Hallek, M.; Jacob, C.; Herling, M. Targeting the disturbed redox equilibrium in chronic lymphocytic leukemia by novel reactive oxygen species-catalytic ‘sensor/effector’ compounds. Leuk. Lymphoma 2011, 52, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, S.; Shaaban, S.; Ba, L.A.; Burkholz, T.; Schneider, T.; Diesel, B.; Kiemer, A.K.; Roseler, A.; Becker, K.; Reichrath, J.; et al. Exploring synthetic avenues for the effective synthesis of selenium- and tellurium-containing multifunctional redox agents. Org. Biomol. Chem. 2009, 7, 4753–4762. [Google Scholar] [CrossRef] [PubMed]
- Jardim, G.A.M.; Lima, D.J.B.; Valenca, W.O.; Lima, D.J.B.; Cavalcanti, B.C.; Pessoa, C.; Rafique, J.; Braga, A.L.; Jacob, C.; da Silva Junior, E.N.; et al. Synthesis of selenium-quinone hybrid compounds with potential antitumor activity via Rh-catalyzed C–H bond activation and click reactions. Molecules 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Bannwitz, S.; Krane, D.; Vortherms, S.; Kalin, T.; Lindenschmidt, C.; Golpayegani, N.Z.; Tentrop, J.; Prinz, H.; Muller, K. Synthesis and structure-activity relationships of lapacho analogues. 2. Modification of the basic naphtho[2,3-b]furan-4,9-dione, redox activation, and suppression of human keratinocyte hyperproliferation by 8-hydroxynaphtho[2,3-b]thiophene-4,9-diones. J. Med. Chem. 2014, 57, 6226–6239. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Sellmer, A.; Wiegrebe, W. Potential antipsoriatic agents: Lapacho compounds as potent inhibitors of HaCaT cell growth. J. Nat. Prod. 1999, 62, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Muller, K. Current status and recent developments in anthracenone antipsoriatics. Curr. Pharm. Des. 2000, 6, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Tellurium. Available online: http://www.ghosttowns.com/states/co/tellurium.html (accessed on 2 February 2018).
- Marut, W.K.; Kavian, N.; Servettaz, A.; Nicco, C.; Ba, L.A.; Doering, M.; Chereau, C.; Jacob, C.; Weill, B.; Batteux, F. The organotelluride catalyst (PHTE)2NQ prevents HOCl-induced systemic sclerosis in mouse. J. Investig. Dermatol. 2012, 132, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Makarovsky, D.; Kalechman, Y.; Sonino, T.; Freidkin, I.; Teitz, S.; Albeck, M.; Weil, M.; Geffen-Aricha, R.; Yadid, G.; Sredni, B. Tellurium compound AS101 induces PC12 differentiation and rescues the neurons from apoptotic death. Ann. N. Y. Acad. Sci. 2003, 1010, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Arsenyan, P.; Vasiljeva, J.; Belyakov, S.; Liepinsh, E.; Petrova, M. Fused selenazolinium salt derivatives with a Se–N+ bond: Preparation and properties. Eur. J. Org. Chem. 2015, 2015, 5842–5855. [Google Scholar] [CrossRef]
- Mániková, D.; Šestáková, Z.; Rendeková, J.; Vlasáková, D.; Lukáčová, P.; Paegle, E.; Arsenyan, P.; Chovanec, M. Resveratrol-inspired benzo[b]selenophenes act as anti-oxidants in yeast. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Jawad Nasim, M.; Ali, W.; Dominguez-Alvarez, E.; da Silva Junior, E.N.; Saleem, R.S.Z.; Jacob, C. Chapter 10 reactive selenium species: Redox modulation, antioxidant, antimicrobial and anticancer activities. In Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; The Royal Society of Chemistry: London, UK, 2018; pp. 277–302. [Google Scholar]
- Dominguez-Alvarez, E.; Gajdacs, M.; Spengler, G.; Palop, J.A.; Marc, M.A.; Kiec-Kononowicz, K.; Amaral, L.; Molnar, J.; Jacob, C.; Handzlik, J.; et al. Identification of selenocompounds with promising properties to reverse cancer multidrug resistance. Bioorg. Med. Chem. Lett. 2016, 26, 2821–2824. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Alvarez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Jacob, C.; Palop, J.A.; Sanmartin, C. Synthesis and antiproliferative activity of novel selenoester derivatives. Eur. J. Med. Chem. 2014, 73, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Prim, D.; Joseph, D.; Kirsch, G. Synthesis of new 2,5-diaryl selenophenes. Phosphorus Sulfur Silicon Relat. Elem. 1994, 91, 137–143. [Google Scholar] [CrossRef]
- Kirsch, G.; Cagniant, P.; Cagniant, D.; Backes, C. The aryl acetic acids new synthons for 3-aryl heterocyclic sulfur coumpounds and their analogs in the selenium and tellurium series. Phosphorus Sulfur Silicon Relat. Elem. 1979, 6, 161. [Google Scholar] [CrossRef]
- Pariagh, S.; Tasker, K.M.; Fry, F.H.; Holme, A.L.; Collins, C.A.; Okarter, N.; Gutowski, N.; Jacob, C. Asymmetric organotellurides as potent antioxidants and building blocks of protein conjugates. Org. Biomol. Chem. 2005, 3, 975–980. [Google Scholar] [PubMed]
- Viswanathan, U.M.; Burkholz, T.; Jacob, C. Electrochemistry at the edge of reason: Chalcogen-based redox systems in biochemistry and drug design. Z. Phys. Chem. 2013, 227, 691–706. [Google Scholar] [CrossRef]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 616, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Flohe, L.; Gunzler, W.A. Assays of glutathione-peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [PubMed]
- Lee, S.; Park, Y.; Kim, J.; Han, S.J. A fluorescence-based assay for measuring the redox potential of 5-lipoxygenase inhibitors. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Z.; Bian, Y.Z.; Furuya, F.; Liu, W.; Choi, M.T.M.; Kobayashi, N.; Li, H.W.; Yang, Q.C.; Mak, T.C.W.; Ng, D.K.P. Synthesis, structure, spectroscopic properties, and electrochemistry of rare earth sandwich compounds with mixed 2,3-naphthalocyaninato and octaethylporphyrinato ligands. Chem. Eur. J. 2001, 7, 5059–5069. [Google Scholar] [CrossRef]
- Waszczak, C.; Akter, S.; Eeckhout, D.; Persiau, G.; Wahni, K.; Bodra, N.; Van Molle, I.; De Smet, B.; Vertommen, D.; Gevaert, K.; et al. Sulfenome mining in arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 11545–11550. [Google Scholar] [CrossRef] [PubMed]
- Thamsen, M.; Jakob, U. The redoxome proteomic analysis of cellular redox networks. Curr. Opin. Chem. Biol. 2011, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Warringer, J.; Anevski, D.; Liu, B.; Blomberg, A. Chemogenetic fingerprinting by analysis of cellular growth dynamics. BMC Chem. Biol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Muthukumar, Y.; Hinkelmann, B.; Franke, R.; Doring, M.; Jacob, C.; Sasse, F. Deciphering intracellular targets of organochalcogen based redox catalysts. Med. Chem. Commun. 2012, 3, 784–787. [Google Scholar] [CrossRef]
- Doering, M.; Diesel, B.; Gruhlke, M.C.H.; Viswanathan, U.M.; Manikova, D.; Chovanec, M.; Burkholz, T.; Slusarenko, A.J.; Kiemer, A.K.; Jacob, C. Selenium- and tellurium-containing redox modulators with distinct activity against macrophages: Possible implications for the treatment of inflammatory diseases. Tetrahedron 2012, 68, 10577–10585. [Google Scholar] [CrossRef]
- Allah, D.R.; Schwind, L.; Abu Asali, I.; Nasim, J.; Jacob, C.; Gotz, C.; Montenarh, M. A scent of therapy: Synthetic polysulfanes with improved physico-chemical properties induce apoptosis in human cancer cells. Int. J. Oncol. 2015, 47, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Saidu, N.E.B.; Touma, R.; Abu Asali, I.; Jacob, C.; Montenarh, M. Diallyl tetrasulfane activates both the eiF2 alpha and Nrf2/HO-1 pathways. Biochim. Biophys. Acta 2013, 1830, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Saidu, N.E.B.; Abu Asali, I.; Czepukojc, B.; Seitz, B.; Jacob, C.; Montenarh, M. Comparison between the effects of diallyl tetrasulfide on human retina pigment epithelial cells (ARPE-19) and HCT116 cells. Biochim. Biophys. Acta 2013, 1830, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Yagdi Efe, E.; Mazumder, A.; Lee, J.Y.; Gaigneaux, A.; Radogna, F.; Nasim, M.J.; Christov, C.; Jacob, C.; Kim, K.W.; Dicato, M.; et al. Tubulin-binding anticancer polysulfides induce cell death via mitotic arrest and autophagic interference in colorectal cancer. Cancer Lett. 2017, 410, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Marut, W.; Jamier, V.; Kavian, N.; Servettaz, A.; Winyard, P.G.; Eggleton, P.; Anwar, A.; Nicco, C.; Jacob, C.; Chereau, C.; et al. The natural organosulfur compound dipropyltetrasulfide prevents HOCl-induced systemic sclerosis in the mouse. Arthritis Res. Ther. 2013, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, Y.; Yabu, T.; Yamashita, M. Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J. Boil. Chem. 2010, 1, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.; Sarfraz, M.; Hartmann, S.F.; Pinnapireddy, S.R.; Nasim, M.J.; Bakowsky, U.; Keck, C.M.; Jacob, C. Resuspendable powders of lyophilized chalcogen particles with activity against microorganisms. Antioxidants 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Estevam, E.C.; Griffin, S.; Nasim, M.J.; Denezhkin, P.; Schneider, R.; Lilischkis, R.; Dominguez-Alvarez, E.; Witek, K.; Latacz, G.; Keck, C.; et al. Natural selenium particles from Staphylococcus carnosus: Hazards or particles with particular promise? J. Hazard. Mater. 2017, 324, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Faulstich, L.; Griffin, S.; Nasim, M.J.; Masood, M.I.; Ali, W.; Alhamound, S.; Omran, Y.; Kim, H.; Kharma, A.; Schafer, K.H.; et al. Nature’s hat-trick: Can we use sulfur springs as ecological source for materials with agricultural and medical applications? Int. Biodeterior. Biodegrad. 2017, 119, 678–686. [Google Scholar] [CrossRef]
- Schneider, T.; Baldauf, A.; Ba, L.A.; Jamier, V.; Khairan, K.; Sarakbi, M.B.; Reum, N.; Schneider, M.; Roseler, A.; Becker, K.; et al. Selective antimicrobial activity associated with sulfur nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 395–405. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Enzyme (±Substrate) | Small Molecule Catalyst (Mimic) | Hybrid of Small Molecule Catalyst and Protein |
|---|---|---|---|
| Origin | Natural | Artificial | Combination |
| Molecular weight | High (well above 10 to 100 kDa) | Low (100–300 Da) | High |
| Stability | Sensitive to degradation | Generally stable | Intermediate |
| Administration | Usually intravenously | Oral administration feasible | Usually intravenously |
| Immune response | Common if administered intravenously | Rare | Possible but avoidable |
| Selectivity for substrates | Highly selective for specific substrates | May use various substrates | May use various substrates |
| Selectivity for pre-existing biochemical signatures | Can be selective if substrate present | Can be selective if substrate present | Can be selective if substrate present |
| Active transport to cellular targets | Usually not selective | Not selective | Possible if antibodies are involved |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ney, Y.; Jawad Nasim, M.; Kharma, A.; Youssef, L.A.; Jacob, C. Small Molecule Catalysts with Therapeutic Potential. Molecules 2018, 23, 765. https://doi.org/10.3390/molecules23040765
Ney Y, Jawad Nasim M, Kharma A, Youssef LA, Jacob C. Small Molecule Catalysts with Therapeutic Potential. Molecules. 2018; 23(4):765. https://doi.org/10.3390/molecules23040765
Chicago/Turabian StyleNey, Yannick, Muhammad Jawad Nasim, Ammar Kharma, Lama A. Youssef, and Claus Jacob. 2018. "Small Molecule Catalysts with Therapeutic Potential" Molecules 23, no. 4: 765. https://doi.org/10.3390/molecules23040765