Cell Penetrating Capacity and Internalization Mechanisms Used by the Synthetic Peptide CIGB-552 and Its Relationship with Tumor Cell Line Sensitivity
Abstract
:1. Introduction
2. Results
2.1. Cell Penetrating Capacity of CIGB-552 and Internalization Mechanisms Involved
2.2. Evaluation of Phosphatidylserine as a Transduction Marker
2.3. COMMD1 Localization and Expression
2.4. In Situ Interaction between COMMD1 and CIGB-552
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Peptide Synthesis
4.3. Cell Line and Culture Medium
4.4. Internalization Assay
4.5. Rab5A and Anti-COMMD1 Immunodetection
4.6. Protein Complementation Assay (PCA)
4.7. Annexin V
4.8. Confocal Microscopy and Image Analysis
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Madani, F.; Lindberg, S.; Langel, Ü.; Futaki, S.; Gräslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 Years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Fernández Massó, J.R.; Oliva Argüelles, B.; Tejeda, Y.; Astrada, S.; Garay, H.; Reyes, O.; Delgado-Roche, L.; Bollati-Fogolín, M.; Vallespí, M.G. The Antitumor Peptide CIGB-552 Increases COMMD1 and Inhibits Growth of Human Lung Cancer Cells. J. Amino Acids 2013, 2013, 251398. [Google Scholar] [CrossRef] [PubMed]
- Vallespi, M.G.; Fernandez, J.R.; Torrens, I.; Garcia, I.; Garay, H.; Mendoza, O.; Granadillo, M.; Falcon, V.; Acevedo, B.; Ubieta, R.; et al. Identification of a novel antitumor peptide based on the screening of an Ala-library derived from the LALF32-51 region. J. Pept. Sci. 2010, 16, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Vallespí, M.G.; Pimentel, G.; Cabrales-Rico, A.; Garza, B.; Oliva, J.; Mendoza, O.; Gomez, Y.; Basaco, T.; Sánchez, I.; Calderón, C.; et al. Antitumor efficacy, pharmacokinetic and biodistribution studies of the anticancer peptide CIGB-552 in mouse models. J. Pept. Sci. 2014, 20, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, P.; Hofker, M.H.; Van De Sluis, B. Biochimica et Biophysica Acta Tuning NF- κB activity: A touch of COMMD proteins. BBA Mol. Basis Dis. 2013, 1832, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Ter-avetisyan, G.; Tu, G.; Nowak, D.; Nitschke, M.; Herrmann, A.; Drab, M.; Cardoso, M.C. Cell Entry of Arginine-rich Peptides Is Independent of endocytosis. J. Biol. Chem. 2009, 284, 3370–3378. [Google Scholar] [CrossRef] [PubMed]
- Astrada, S.; Gomez, Y.; Barrera, E.; Obal, G.; Pritsch, O.; Pantano, S.; Vallespí, M.G.; Bollati-Fogolín, M. Comparative analysis reveals amino acids critical for anticancer activity of peptide CIGB-552. J. Pept. Sci. 2016, 22, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Jobin, M.L.; Alves, I.D. On the importance of electrostatic interactions between cell penetrating peptides and membranes: A pathway toward tumor cell selectivity? Biochimie 2014, 107, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Tünnemann, G.; Ter-Avetisyan, G.; Martin, R.M.; Stöckl, M.; Herrmann, A.; Cardoso, M.C. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J. Pept. Sci. 2008, 14, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Duchardt, F.; Fotin-Mleczek, M.; Schwarz, H.; Fischer, R.; Brock, R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic 2007, 8, 848–866. [Google Scholar] [CrossRef] [PubMed]
- Bucci, C.; Parton, R.G.; Mather, I.H.; Stunnenberg, H.; Simons, K.; Hoflack, B.; Zerial, M. The small GTPase rab5 function as a regulatory factor in the early endocytic pathway. Cell 1992, 70, 715–728. [Google Scholar] [CrossRef]
- Bucci, C.; Lütcke, A.; Steele-Mortimer, O.; Olkkonen, V.M.; Dupree, P.; Chiariello, M.; Bruni, C.B.; Simons, K.; Zerial, M. Co-operative regulation of endocytosis by three RAB5 isoforms. FEBS Lett. 1995, 366, 65–71. [Google Scholar] [CrossRef]
- Dutta, D.; Donaldson, J.G. Search for inhibitors of endocytosis. Cell. Logist. 2012, 2, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The use of inhibitors to study endocytic pathways of gene carriers: Optimization and pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Misiak, L.E.; Jarosz-Wilkołazka, A.; Zarzyka, B.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Biophysical characterization of genistein-membrane interaction and its correlation with biological effect on cells—The case of EYPC liposomes and human erythrocyte membranes. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Rinner, B.; Asslaber, M.; Schaider, H.; Walzer, S.; Novak, A.; Lohner, K.; Zweytick, D. In search of a novel target—Phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.D.; Blanco, V.M.; Sulaiman, M.K.; Vallabhapurapu, S.L.; Chu, Z.; Franco, R.S.; Qi, X. Variation in human cancer cell external phosphatidylserine is regulated by flippase activity and intracellular calcium. Oncotarget 2015, 6, 34375–34388. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Morgan, C.T.; Shinde, U.; Haddock, G.; Lutsenko, S. COMMD1 Forms Oligomeric Complexes Targeted to the Endocytic Membranes via Specific Interactions with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2009, 284, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, W.B.; Fuselier, T.; He, J.; Wimley, W.C. Mechanism Matters: A Taxonomy of Cell Penetrating Peptides. Trends Biochem. Sci. 2015, 40, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.B.; Pereira, M.P.; Kelley, S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Melo, M.N.; Ferre, R.; Castanho, M.A.R.B. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Pellois, J.P. Peptide translocation through the plasma membrane of human cells: Can oxidative stress be exploited to gain better intracellular access? Commun. Integr. Biol. 2016, 9, e1205771. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef] [PubMed]
- de Chaumont, F.; Dallongeville, S.; Chenouard, N.; Hervé, N.; Pop, S.; Provoost, T.; Meas-Yedid, V.; Pankajakshan, P.; Lecomte, T.; Le Montagner, Y.; et al. Icy: An open bioimage informatics platform for extended reproducible research. Nat. Methods 2012, 9, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Thibeaux, R.; Labruyère, E.; Guillén, N.; Olivo-Marin, J.C. 3-D active meshes: Fast discrete deformable models for cell tracking in 3-D time-lapse microscopy. IEEE Trans. Image Process. 2011, 20, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are available from the authors upon the signature of a material transfer agreement (MTA). |


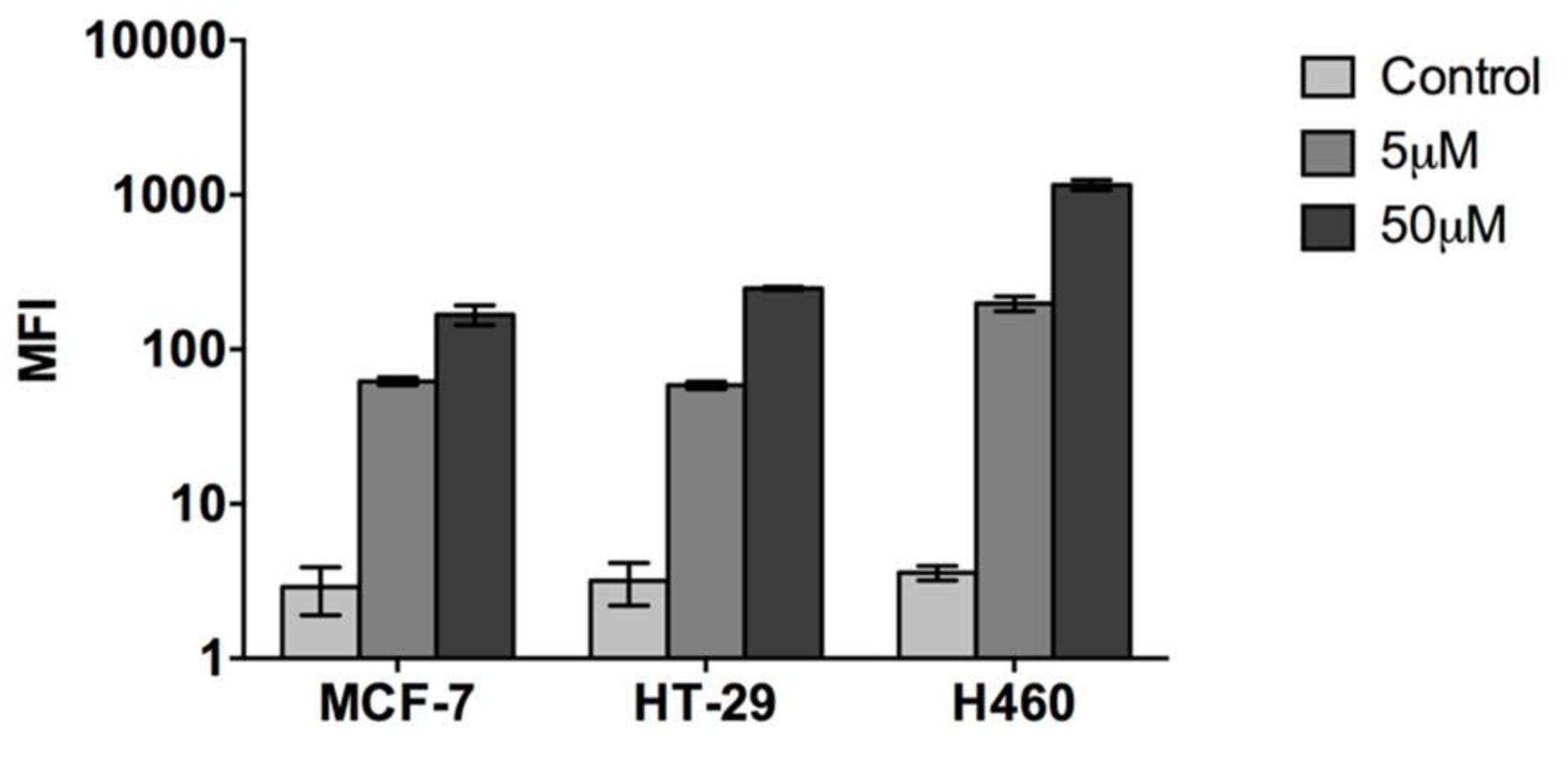





| Peptide | Sequence | H460 | HT-29 | MCF-7 |
|---|---|---|---|---|
| CIGB-552 | Ac-HARIKpTFRRlKWKYKGKFW | 23 ± 8 | 166 ± 66 | 338 ± 39 |
| Peptide | Sequence |
|---|---|
| L2 | HARIKPTFRRLKWKYKGKFW |
| CIGB-552 | Ac-HARIKpTFRRlKWKYKGKFW |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astrada, S.; Fernández Massó, J.R.; Vallespí, M.G.; Bollati-Fogolín, M. Cell Penetrating Capacity and Internalization Mechanisms Used by the Synthetic Peptide CIGB-552 and Its Relationship with Tumor Cell Line Sensitivity. Molecules 2018, 23, 801. https://doi.org/10.3390/molecules23040801
Astrada S, Fernández Massó JR, Vallespí MG, Bollati-Fogolín M. Cell Penetrating Capacity and Internalization Mechanisms Used by the Synthetic Peptide CIGB-552 and Its Relationship with Tumor Cell Line Sensitivity. Molecules. 2018; 23(4):801. https://doi.org/10.3390/molecules23040801
Chicago/Turabian StyleAstrada, Soledad, Julio Raúl Fernández Massó, Maribel G. Vallespí, and Mariela Bollati-Fogolín. 2018. "Cell Penetrating Capacity and Internalization Mechanisms Used by the Synthetic Peptide CIGB-552 and Its Relationship with Tumor Cell Line Sensitivity" Molecules 23, no. 4: 801. https://doi.org/10.3390/molecules23040801
APA StyleAstrada, S., Fernández Massó, J. R., Vallespí, M. G., & Bollati-Fogolín, M. (2018). Cell Penetrating Capacity and Internalization Mechanisms Used by the Synthetic Peptide CIGB-552 and Its Relationship with Tumor Cell Line Sensitivity. Molecules, 23(4), 801. https://doi.org/10.3390/molecules23040801






