3,3?-Bicarbazole-Based Host Molecules for Solution-Processed Phosphorescent OLEDs
Abstract
:1. Introduction
2. Results and Discussions
2.1. Synthesis and Thermal Analysis
2.2. Photophyscial and Electrochemical Properties
2.3. Solution-Processed OLEDs
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
3.2.1. 3,3′-bicarbazole (BCz)
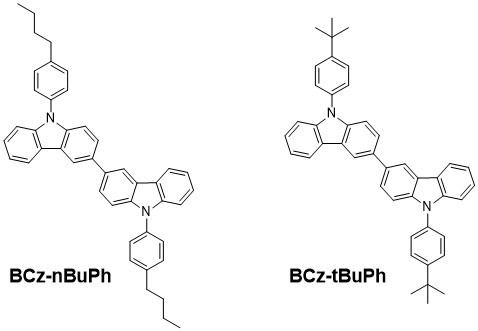
3.2.2. 9,9′-di-4-n-butylphenyl-9H,9′H-3,3′-bicarbazole (BCz-nBuPh)
3.2.3. 9,9′-di-4-t-butylphenyl-9H,9′H-3,3′-bicarbazole (BCz-tBuPh)
3.3. Device Fabrication and Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Park, S.Y. Phosphorescent iridium(III) complexes: Toward high phosphorescence quantum efficiency through ligand control. Dalton Trans. 2009, 1267–1282. [Google Scholar]
- Murawski, C.; Leo, K.; Gather, M.C. Efficiency Roll-Off in Organic Light-Emitting Diodes. Adv. Mater. 2013, 25, 6801–6827. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.H.; Kumar, S.; Agrawal, A.; Li, T.H.; Sahoo, S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C 2015, 3, 2974–3002. [Google Scholar] [CrossRef]
- Yang, T.; Xu, H.; Zhao, B.; Tao, P.; Sun, P.; Wang, H.; Xu, B.; Wong, W. Three carbazole-based host materials: facile synthesis, photophysical properties and performances in PhOLED. Tetrahedron 2016, 72, 8066–8072. [Google Scholar] [CrossRef]
- Baldo, M.A.; Lamansky, S.; Burrows, P.E.; Thompson, M.E.; Forrest, S.R. Very High-Efficiency Green Organic Light-Emitting Devices Based on Electrophosphorescence. Appl. Phys. Lett. 1999, 75, 4–6. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thanikachalam, V.; Sathishkumar, R. Highly Phosphorescent Green Emitting Iridium(III) Complexes for Application in OLEDs. New J. Chem. 2015, 39, 235–245. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Cho, Y.J.; Aziz, H. Exciton-Induced Degradation of Carbazole-Based Host Materials and Its Role in the Electroluminescence Spectral Changes in Phosphorescent Organic Light Emitting Devices with Electrical Aging. ACS Appl. Mater. Interfaces 2017, 9, 14145–14152. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, H.; Toyota, N.; Nakanishi, H.; Ishizaka, T.; Pu, Y.J.; Kido, J. 3,3′-Bicarbazole-Based Host Materials for High-Efficiency Blue Phosphorescent OLEDs with Extremely Low Driving Voltage. Adv. Mater. 2012, 24, 3212–3217. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Hong, Y.-H.; Chang, C.-H.; Su, H.-C.; Wu, C.C.; Matoliukstyte, A.; Simokaitiene, J.; Grigalevicius, S.; Grazulevicius, J.V.; Hsu, C.-P. 3-(9-Carbazolyl)carbazoles and 3,6-Di(9-carbazolyl)-carbazoles as Effective Host Materials for Efficient Blue Organic Electrophosphorescence. Adv. Mater. 2007, 19, 862–866. [Google Scholar] [CrossRef]
- Ameen, S.; Lee, J.; Han, H.; Suh, M.C.; Lee, C. Curing Temperature Reduction and Performance Improvement of Solution processable Hole-transporting Materials for Phosphorescent OLEDs by Manipulation of Cross-linking Functionalities and Core Structures. RSC Adv. 2016, 6, 33212–33220. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, S.K.; Hwang, S.-H.; Lee, S.-S.; Yu, E.; Lee, J.Y. Correlation of Molecular Structure with Photophysical Properties and Device Performances of Thermally Activated Delayed Fluorescent Emitters. J. Phys. Chem. C 2016, 120, 2485–2493. [Google Scholar] [CrossRef]
- Grybauskaite-Kaminskiene, G.; Ivaniuk, K.; Bagdziunas, G.; Turyk, P.; Stakhira, P.; Baryshnikov, G.; Volyniuk, D.; Cherpak, V.; Minaev, B.; Hotra, Z.; et al. Contribution of TADF and exciplex emission for efficient “warm-white” OLEDs. J. Mater. Chem. C 2018, 6, 1543–1550. [Google Scholar] [CrossRef]
- Ahn, S.I.; Kim, W.K.; Ryu, S.H.; Kim, K.J.; Lee, S.E.; Kim, S.H.; Park, J.C.; Choi, K.C. OLED with a Controlled Molecular Weight of the PVK (Poly(9-vinylcarbazole)) Formed by a Reactive Ink-Jet Process. Org. Electron. 2012, 13, 980–984. [Google Scholar] [CrossRef]
- Choulis, S.A.; Choong, V.-E.; Mathai, M.K.; So, F. The effect of interfacial layer on the performance of organic light-emitting diodes. Appl. Phys. Lett. 2005, 87, 113503. [Google Scholar] [CrossRef]
- Yook, K.S.; Lee, J.Y. Small Molecule Host Materials for Solution Processed Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2014, 26, 4218–4233. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Xiao, T.; Hellerich, E.; Chen, Y.; Shinar, R.; Shinar, J. High-Efficiency Solution-Processed Small Molecule Electrophosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2011, 23, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hlil, A.; Wang, J.; Chen, K.; Hay, A. Synthesis of Homo- and Copoly(arylene bicarbazole)s via Nucleophilic Substitution Polycondensation Reactions of NH Groups with Activated Dihalides. Macromolecules 2007, 40, 4744–4746. [Google Scholar] [CrossRef]
- Zhang, G.; Auer-Berger, M.; Gehrig, D.W.; Blom, P.W.M.; Baumgarten, M.; Schollmeyer, D.; List-Kratochvil, E.J.W.; Müllen, K. Blue Light Emitting Polyphenylene Dendrimers with Bipolar Charge Transport Moieties. Molecules 2016, 21, 1400. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qin, J.; Ren, Z.; Peng, Q.; Xie, G.; Li, Z. Pyrene-Based Blue AIEgen: Enhanced Hole Mobility and Good EL Performance in Solution-Processed OLEDs. Molecules 2017, 22, 2144. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, R.; Mena-Osteritz, E.; Walzer, K.; Pfeiffer, M.; Bäuerle, P. A–D–A-Type Oligothiophenes for Small Molecule Organic Solar Cells: Extending the π-System by Introduction of Ring-Locked Double Bonds. Adv. Funct. Mater. 2015, 25, 1845–1856. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.-K.; Zheng, C.-J.; Ye, J.; Liu, C.-L.; Li, F.; Ou, X.-M.; Lee, C.-S.; Zhang, X.-H. High Performance Exciplex-Based Fluorescence−Phosphorescence White Organic Light-Emitting Device with Highly Simplified Structure. Chem. Mater. 2015, 27, 5206–5211. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Chen, R.; Xie, G.; Zheng, C.; Huang, W. Carbazole/Oligofluorene End-Capped Hexanes: Solution-processable Host Materials for Phosphorescent Organic Light-Emitting Diodes. J. Mater. Chem. C 2017, 5, 4442–4447. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lin, H.-W.; Mondal, E.; Wong, K.-T. Efficient Solution-Processed Green and White Phosphorescence Organic Light-Emitting Diodes Based on Bipolar Host Materials. Org. Electron. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Bagdžiūnas, G.; Grybauskaitė, G.; Kostiv, N.; Ivaniuk, K.; Volyniuk, D.; Lazauskas, A. Green and red phosphorescent organic light-emitting diodes with ambipolar hosts based on phenothiazine and carbazole moieties: Photoelectrical properties, morphology and efficiency. RSC Adv. 2016, 6, 61544–61554. [Google Scholar]
- Reig, M.; Bagdziunas, G.; Volyniuk, D.; Grazulevicius, J.V.; Velasco, D. Tuning the ambipolar charge transport properties of tricyanovinyl-substituted carbazole-based materials. Phys. Chem. Chem. Phys. 2017, 19, 6721–6730. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| Host Materials | Tm/ Tg a (oC) | Td b (oC) | λmax, solution (nm) UV-vis, PL | λmax, film (nm) UV-vis, PL | Eg c (eV) | Eox, onset d (V versus Fc/Fc+) | IP e (eV) | EA f (eV) |
|---|---|---|---|---|---|---|---|---|
| BCz-nBuPh | 146/58 | 410 | 305, 409 | 308, 413 | 3.32 | 0.45 | 5.55 | 2.13 |
| BCz-tBuPh | 295/136 | 423 | 304, 409 | 308, 411 | 3.32 | 0.45 | 5.55 | 2.13 |
| Host Materials | λEL (nm) | CIE (x, y) a | V1 , V1000 (V) b | Lmax c (cd/m2) | CE max, 1000 (cd/A) d | PE max, 1000 (lm/W) e | EQE max, 1000 (%) f |
|---|---|---|---|---|---|---|---|
| BCz-nBuPh | 510 | (0.301, 0.620) | 3.3, 5.8 | 6598 | 30.8, 27.6 | 28.1, 14.9 | 8.8. 7.9 |
| BCz-tBuPh | 510 | (0.294, 0.621) | 3.1, 4.9 | 8372 | 43.1, 38.8 | 40.0, 24.9 | 12.5. 11.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, S.; Lee, J.; Lim, E.; Jung, B.J. 3,3?-Bicarbazole-Based Host Molecules for Solution-Processed Phosphorescent OLEDs. Molecules 2018, 23, 847. https://doi.org/10.3390/molecules23040847
Kim J, Lee S, Lee J, Lim E, Jung BJ. 3,3?-Bicarbazole-Based Host Molecules for Solution-Processed Phosphorescent OLEDs. Molecules. 2018; 23(4):847. https://doi.org/10.3390/molecules23040847
Chicago/Turabian StyleKim, Jungwoon, Suhan Lee, Jaemin Lee, Eunhee Lim, and Byung Jun Jung. 2018. "3,3?-Bicarbazole-Based Host Molecules for Solution-Processed Phosphorescent OLEDs" Molecules 23, no. 4: 847. https://doi.org/10.3390/molecules23040847
APA StyleKim, J., Lee, S., Lee, J., Lim, E., & Jung, B. J. (2018). 3,3?-Bicarbazole-Based Host Molecules for Solution-Processed Phosphorescent OLEDs. Molecules, 23(4), 847. https://doi.org/10.3390/molecules23040847