Study on the Bactericidal Mechanism of Atmospheric-Pressure Low-Temperature Plasma against Escherichia coli and Its Application in Fresh-Cut Cucumbers
Abstract
1. Introduction
2. Results
2.1. The Bactericidal Mechanism
2.1.1. SEM Observation
2.1.2. FM Observation
2.1.3. Evaluation of Conductivity
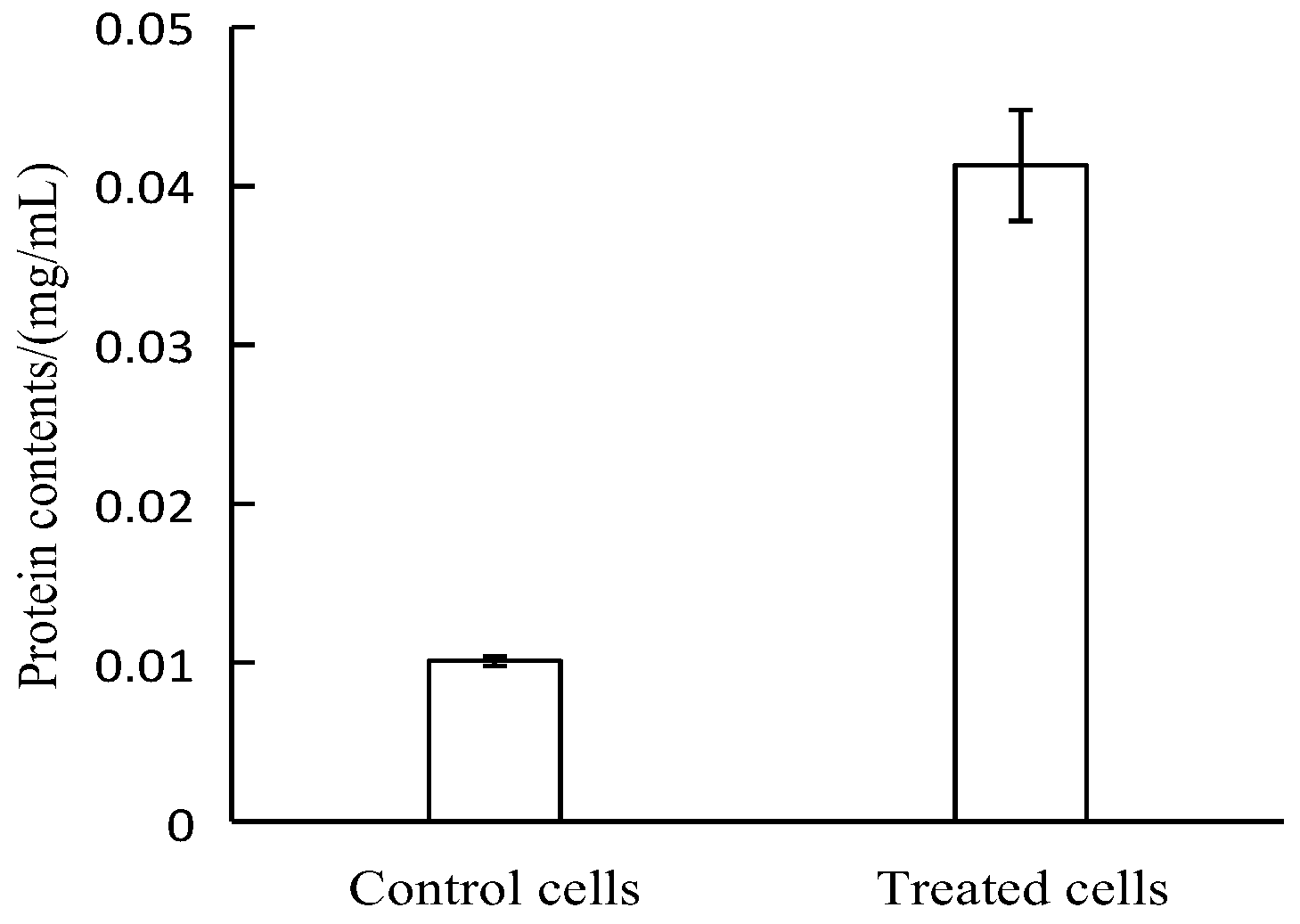
2.1.4. Determination of Protein Concentration
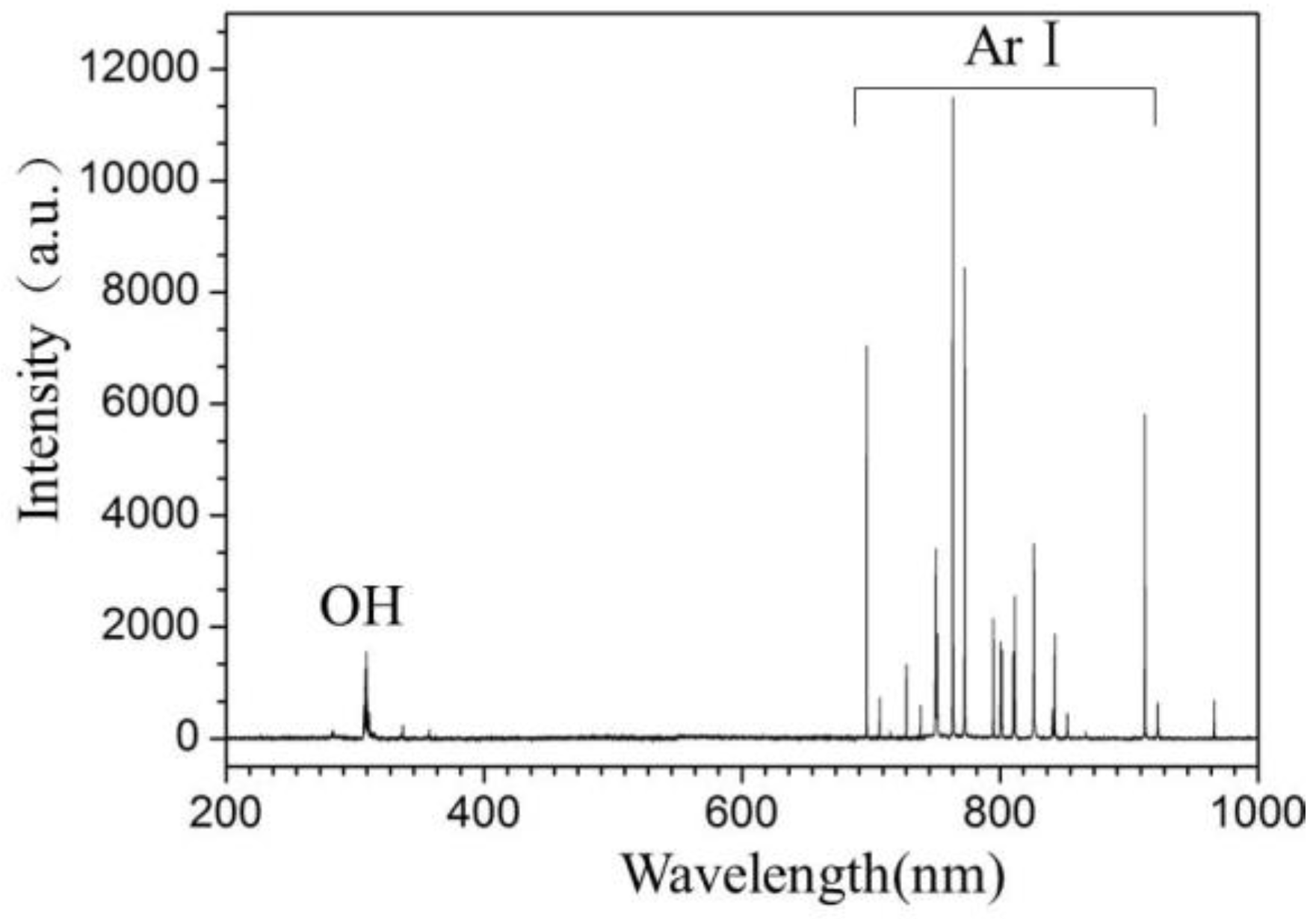
2.1.5. Optical Emission Spectrum
2.2. The Application of APLTP to Sterilize Fresh-Cut Cucumbers
2.2.1. Effect of APLTP Treatment on the Physicochemical Properties of Fresh-Cut Cucumber
2.2.2. Effect of APLTP Treatment on Sensory Indicators of Fresh-Cut Cucumber
3. Discussion
4. Experimental Section
4.1. Sterilization Mechanism
4.1.1. Experimental Apparatus
4.1.2. Preparation of Inoculum
4.1.3. SEM Observation
4.1.4. FM Observation
4.1.5. Determination of Conductivity
4.1.6. Determination of Protein Concentration
4.1.7. Optical Emission Spectroscopy
4.2. The Application of APLTP for Sterilization of Fresh-Cut Cucumber
4.2.1. APLTP Treatment
4.2.2. Influences of the Plasma Treatment on the Physicochemical Properties of the Cut Cucumber
4.2.3. The Influences of the Plasma Treatment on the Sensory Indicators of the Cut Cucumber
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ben, B.Z.; Carrã, G.; Charpentier, E.; Le-Bras, F.; Maho, T.; Robert, E.; Pouvesle, J.M.; Polidor, F.; Gangloff, S.C.; Boudifa, M. Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms. PLoS ONE 2017, 12, e0180183. [Google Scholar]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Woedtke, T.V.; Brandenburg, R.; Hagen, T.V.D.; Weltmann, K. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44, 13002–13019. [Google Scholar] [CrossRef]
- Machala, Z.; Hensel, K.; Akishev, Y. Plasma for Bio-Decontamination, Medicine and Food Security; Springer: Amsterdam, The Netherlands, 2012; pp. 63–94. [Google Scholar]
- Cui, H.; Bai, M.; Yuan, L.; Surendhiran, D.; Lin, L. Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int. J. Food Microbiol. 2018, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Sun, Y.; Wu, H.; Zhu, W.; Lopez, J.L.; Liu, W.; Zhang, J.; Li, R.; Fang, J. Atmospheric pressure cold plasma as an antifungal therapy. Appl. Phys. Lett. 2011, 98, 480. [Google Scholar] [CrossRef]
- Chu, P.K. Recent developments and applications of plasma immersion ion implantation. J. Vac. Sci. Technol. B 2004, 22, 289–296. [Google Scholar] [CrossRef]
- Ma, T.J.; Lan, W.S. Effects of non-thermal plasma sterilization on volatile components of tomato juice. Int. J. Environ. Sci. Technol. 2015, 12, 3767–3772. [Google Scholar] [CrossRef]
- Sen, Y.; Mutlu, M. Sterilization of Food Contacting Surfaces via Non-Thermal Plasma Treatment: A Model Study with Escherichia coli-Contaminated Stainless Steel and Polyethylene Surfaces. Food Bioprocess Technol. 2013, 6, 3295–3304. [Google Scholar] [CrossRef]
- Noriega, E.; Shama, G.; Laca, A.; Díaz, M.; Kong, M.G. Cold atmospheric gas plasma disinfection of chicken meat and chicken skin contaminated with Listeria innocua. Food Microbiol. 2011, 28, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Critzer, F.J.; Kellywintenberg, K.; South, S.L.; Golden, D.A. Atmospheric plasma inactivation of foodborne pathogens on fresh produce surfaces. J. Food Prot. 2007, 70, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Shama, G.; Kong, M.G. Microbial Decontamination of Mango and Melon Surface Using a Cold Atmospheric Plasma Treatment. In Proceedings of the IEEE 34th International Conference on Plasma Science, Albuquerque, NM, USA, 17–22 June 2007; p. 335. [Google Scholar]
- Niemira, B.A.; Sites, J. Cold plasma inactivates Salmonella Stanley and Escherichia coli O157:H7 inoculated on golden delicious apples. J. Food Prot. 2008, 71, 1357. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Shama, G.; Kong, M.G. Cold atmospheric plasma disinfection of cut fruit surfaces contaminated with migrating microorganisms. J. Food Prot. 2008, 71, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Yun, G. The sterilization of Escherichia coli by dielectric-barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2011, 257, 7065–7070. [Google Scholar] [CrossRef]
- Deng, X.T.; Shi, J.J.; Shama, G.; Kong, M.G. Effects of microbial loading and sporulation temperature on atmospheric plasma inactivation of Bacillus subtilis spores. Appl. Phys. Lett. 2005, 87, 772. [Google Scholar] [CrossRef]
- Masaoka, S. Plasma sterilization of polyethylene terephthalate bottles by pulsed corona discharge at atmospheric pressure. Biocontrol Sci. 2007, 12, 59–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiong, Z.; Lu, X.P.; Feng, A.; Pan, Y.; Ostrikov, K. Highly effective fungal inactivation in He+O2 atmospheric-pressure nonequilibrium plasmas. Phys. Plasmas 2010, 17, 391. [Google Scholar] [CrossRef]
- Nie, Q.; Cao, Z.; Ren, C.S.; Wang, D.Z.; Kong, M.G. A two-dimensional cold atmospheric plasma jet array for uniform treatment of large-area surfaces for plasma medicine. New J. Phys. 2009, 11, 115015. [Google Scholar] [CrossRef]
- Robert, E.; Darny, T.; Dozias, S.; Iseni, S.; Pouvesle, J.M. New insights on the propagation of pulsed atmospheric plasma streams: From single jet to multi jet arrays. Phys. Plasmas 2015, 22, 194008–194141. [Google Scholar] [CrossRef]
- Babaeva, N.Y.; Kushner, M.J. Intracellular electric fields produced by dielectric barrier discharge treatment of skin. J. Phys. D Appl. Phys. 2010, 43, 2851. [Google Scholar] [CrossRef]
- Bourdon, A.; Darny, T.; Pechereau, F.; Pouvesle, J.M.; Viegas, P.; Iséni, S.; Robert, E. Numerical and experimental study of the dynamics of a μs helium plasma gun discharge with various amounts of N2 admixture. Plasma Sources Sci. Technol. 2016, 25, 035002. [Google Scholar] [CrossRef]
- Obradović, B.M.; Ivković, S.S.; Kuraica, M.M. Spectroscopic measurement of electric field in dielectric barrier discharge in helium. Appl. Phys. Lett. 2008, 92, 2950. [Google Scholar] [CrossRef]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.M.; Pichon, C.; Robert, E. Cold Atmospheric Plasma Parameters Investigation for Efficient Drug Delivery in HeLa Cells. IEEE Technol. Plasma Sci. 2017, 2, 109–115. [Google Scholar] [CrossRef]
- Jinno, M.; Ikeda, Y.; Motomura, H.; Kido, Y.; Satoh, S. Investigation of plasma induced electrical and chemical factors and their contribution processes to plasma gene transfection. Arch. Biochem. Biophys. 2016, 605, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Leduc, M.; Guay, D.; Leask, R.L.; Coulombe, S. Cell permeabilization using a non-thermal plasma. New J. Phys. 2009, 11, 115021. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Guijie, L.I.; Peng, S.; Qiang, W.; Qian, Y.U.; Kai, Z.; Xin, Z. Dendrobium candidum Wall. ex Lindl. attenuates CCl4-induced hepatic damage in imprinting control region mice. Exp. Ther. Med. 2014, 8, 1015–1021. [Google Scholar]
- Won, M.Y.; Lee, S.J.; Min, S.C. Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.M.; Downing, A.; Zhang, J.; Yang, C. Membrane stability of the desert moss Mitt. during desiccation and rehydration. J. Bryol. 2012, 34, 1–8. [Google Scholar] [CrossRef]
- Mirzaee, M.; Moieni, A.; Ghanati, F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napusL.) cultivars. J. Agric. Sci. Technol. 2013, 15, 593–602. [Google Scholar]
- Misra, N.N.; Keener, K.M.; Bourke, P.; Mosnier, J.P.; Cullen, P.J. In-package atmospheric pressure cold plasma treatment of cherry tomatoes. J. Biosci. Bioeng. 2014, 118, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P.J. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Montie, T.C.; Kelly-Wintenberg, K.; Roth, J.R. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Technol. Plasma Sci. 2002, 28, 41–50. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Laroussi, M. Low-Temperature Plasmas for Medicine. IEEE Technol. Plasma Sci. 2009, 37, 714–725. [Google Scholar] [CrossRef]
- Laroussi, M.; Richardson, J.P.; Dobbs, F.C. Effects of nonequilibrium atmospheric pressure plasmas on the heterotrophic pathways of bacteria and on their cell morphology. Appl. Phys. Lett. 2002, 81, 772–774. [Google Scholar] [CrossRef]
- Fernã, N.A.; Noriega, E.; Thompson, A. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 2013, 33, 24–29. [Google Scholar]
- Halfmann, H.; Denis, B.; Bibinov, N.; Wunderlich, J.; Awakowicz, P. Identification of the most efficient VUV/UV radiation for plasma based inactivation of Bacillus atrophaeus spores. J. Phys. D Appl. Phys. 2007, 40, 5907–5911. [Google Scholar] [CrossRef]
- Moisan, M. Plasma Sterilization: Methods and Mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Boudam, M.K.; Moisan, M.; Saoudi, B.; Popovici, C.; Gherardi, N.; Massines, F. Bacterial spore inactivation by atmospheric-pressure plasmas in the presence or absence of UV photons as obtained with the same gas mixture. J. Phys. D Appl. Phys. 2006, 39, 3494. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Indicators | Control | Treated |
|---|---|---|
| Moisture content (%) | 95.135 ± 0.05 a | 95.153 ± 0.05 a |
| Vc (mg/100 g) | 20.25 ± 0.10 a | 20.27 ± 0.10 a |
| Relative conductivity (%) | 9.32 ± 0.10 a | 9.35 ± 0.10 a |
| MDA (μmol/L) | 0.00121 ± 0.00011 a | 0.00125 ± 0.00012 a |
| Soluble solids (Brix) | 3.6 ± 0.10 a | 3.7 ± 0.12 a |
| pH | 6.02 ± 0.10 a | 6.05 ± 0.10 a |
| Indicators | Control | Treated | |
|---|---|---|---|
| Aroma components | (E,Z)-2,6-Nonadienal | 42.53 ± 2.54 a | 40.61 ± 1.11 a |
| (E)-2-Nonenal (%) | 12.54 ± 0.57 a | 11.54 ± 0.58 a | |
| Hexanal (%) | 9.71 ± 0.54 a | 10.64 ± 1.33 a | |
| (E)-2-Hexenal (%) | 4.42 ± 0.28 a | 4.72 ± 0.39 a | |
| (Z,Z)-3,6-Nonadienal (%) | 14.09 ± 0.17 b | 14.59 ± 0.32 a | |
| Color parameters | L * | 63.78 ± 0.25 a | 63.89 ± 0.23 a |
| a * | −6.07 ± 0.10 a | −5.98 ± 0.15 a | |
| b * | 16.61 ± 0.10 a | 16.63 ± 0.13 a | |
| △E | 0 | 0.73 ± 0.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, Z.; Wang, S. Study on the Bactericidal Mechanism of Atmospheric-Pressure Low-Temperature Plasma against Escherichia coli and Its Application in Fresh-Cut Cucumbers. Molecules 2018, 23, 975. https://doi.org/10.3390/molecules23040975
Sun Y, Zhang Z, Wang S. Study on the Bactericidal Mechanism of Atmospheric-Pressure Low-Temperature Plasma against Escherichia coli and Its Application in Fresh-Cut Cucumbers. Molecules. 2018; 23(4):975. https://doi.org/10.3390/molecules23040975
Chicago/Turabian StyleSun, Yan, Zhiwei Zhang, and Shiqing Wang. 2018. "Study on the Bactericidal Mechanism of Atmospheric-Pressure Low-Temperature Plasma against Escherichia coli and Its Application in Fresh-Cut Cucumbers" Molecules 23, no. 4: 975. https://doi.org/10.3390/molecules23040975
APA StyleSun, Y., Zhang, Z., & Wang, S. (2018). Study on the Bactericidal Mechanism of Atmospheric-Pressure Low-Temperature Plasma against Escherichia coli and Its Application in Fresh-Cut Cucumbers. Molecules, 23(4), 975. https://doi.org/10.3390/molecules23040975






