The Effect of PUFA-Rich Plant Oils and Bioactive Compounds Supplementation in Pig Diet on Color Parameters and Myoglobin Status in Long-Frozen Pork Meat
Abstract
:1. Introduction
2. Results
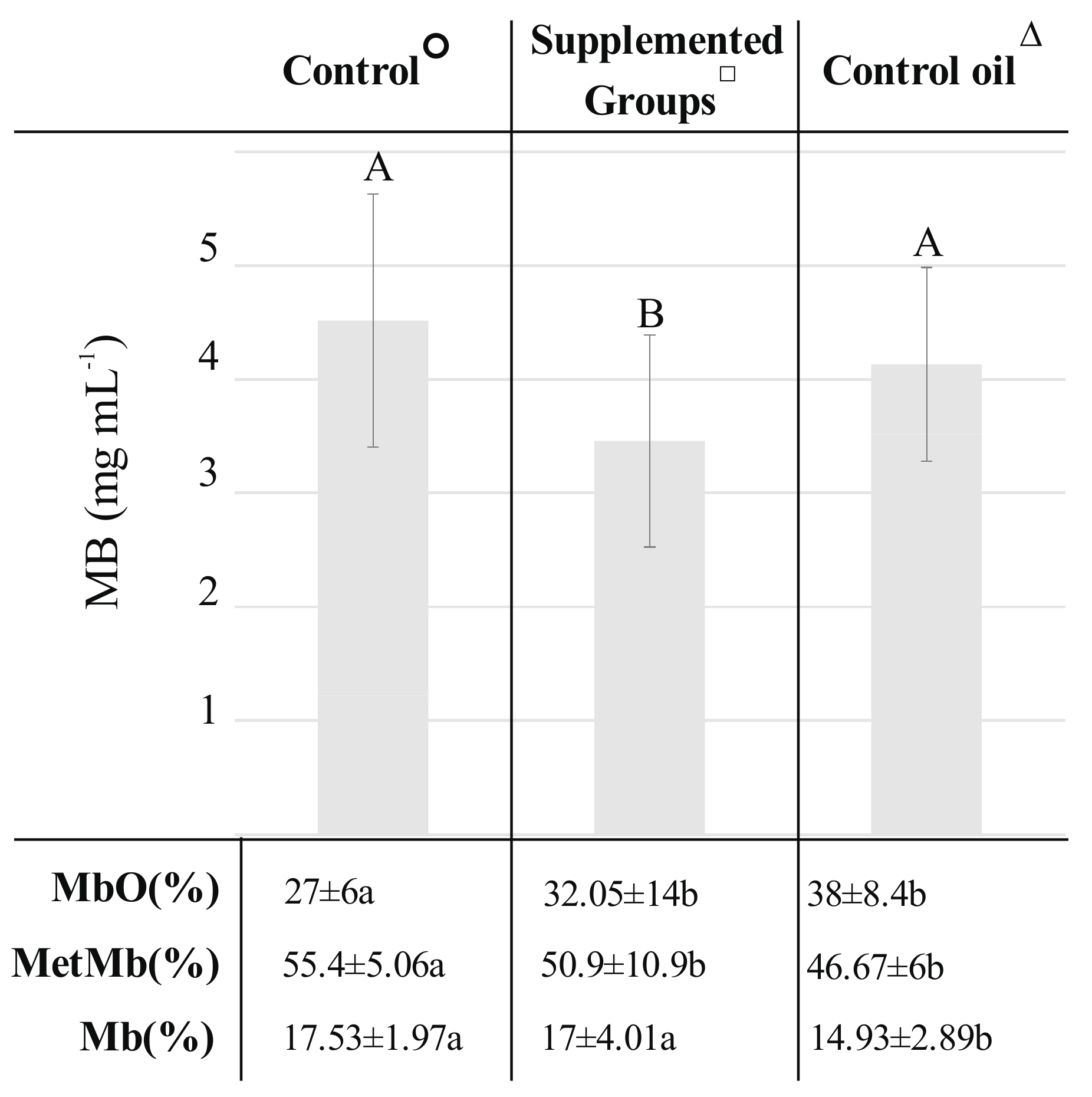
2.1. Differences in Color Components and Myoglobin Content between Meat Obtained from Pigs Fed Fodder with the Addition of Oils Rich in Unsaturated Fatty Acids (UFA) and Oils Rich in UFA + Antioxidants
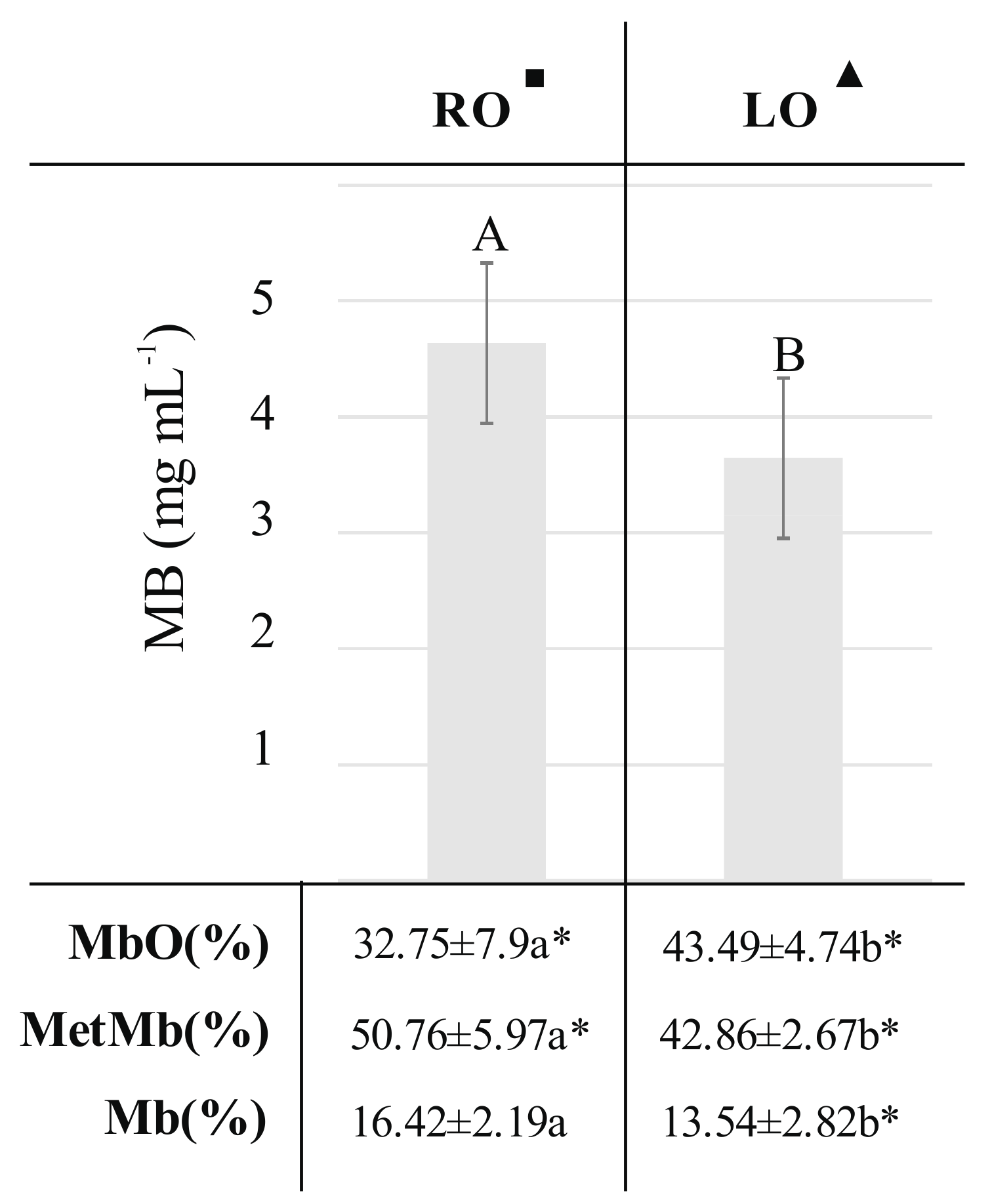
2.2. The Impact of the Type of Plant Oil Addition to Fodder of Pigs on Color Parameters and Myoglobin Content of Pork Semimembranosus m.
2.3. The Effect of an Addition of Different Antioxidants to Pigs’ Fodder on Color Parameters and Myoglobin Content in Pork Meat
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Design and Sample Collection
5.2. pH Measurement
5.3. NIR Measurement
5.4. Color Measurement
5.5. Preparation of MetMb, DeoMb and OxyMb
5.6. Myoglobin Measurement
5.7. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Trait | Control° (n = 6, N = 10) | Control Oil∆ (n = 12, N = 10) | VEˣ (n = 12,N = 10) | Se+ (n = 12, N = 10) | VESe◊ (n = 12, N = 10) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | |
| L* | 45.55 ± 4.52ac | 45.33 (36.82–56.44) | 43.34 ± 3.56b | 43.62 (34.35–54.83) | 45.36 ± 4.74a | 44.75 (35.6–57.26) | 43.83 ± 4.98ac | 43.38 (35.15–58.62) | 45.66 ± 4.74bc | 45.32 (36.66–60.94 |
| a* | 11.87 ± 3.44ac | 11.6 (6.95–19.55) | 14.5 ± 3.5b | 14.3 (5.61–22.46) | 11.01 ± 4.31ac | 10.58 (3.91–24.89) | 12.83 ± 4.22a | 12.57 (5.61–26.52) | 11.95 ± 4.22c | 11.41 (3.02–24.91) |
| b* | 4.53 ± 1.7ac | 4.4 (0.49–8.92) | 5.08 ± 1.99a | 4.75 (1.4–11.47) | 3.61 ± 1.5c | 3.38 (0.33–8.66) | 4.72 ± 2.06b | 4.31 (1.75–12.53) | 4.29 ± 1.81ac | 3.88 (1.14–9.73) |
| C* | 12.82 ± 3.41ac | 12.45 (7.5–21.49) | 15.47 ± 3.61 | 15.27 (6.54–25.22) | 11.61 ± 4.41 | 11.14 (4.05–26.35) | 13.78 ± 4.36 | 13.33 (5.88–29.33) | 12.8 ± 4.28 | 12.32 (5.5–25.68) |
| h° | 0.57 ± 0.14 | 0.36 (0.15–0.77) | 0.34 ± 0.12 | 0.33 (0.12–0.67) | 0.33 ± 0.12 | 0.32 (0.07–0.68) | 0.36 ± 0.13 | 0.34 (0.16–0.69) | 0.36 ± 0.14 | 0.33 (0.1–1.04) |
References
- Verbeke, W.; De Smet, S.; Vackier, I.; Van Oeckel, M.J.; Warnant, S.N.; Van Kenhove, P. Role of intrinsic search cues in the formation of consumer preferences and choice for pork chops. Meat Sci. 2005, 69, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Valous, N.A.; Mendoza, F.; Sun, D.W.; Allen, P. Classification of pre-sliced pork and Turkey ham qualities based on image colour and textural features and their relationships with consumer responses. Meat Sci. 2010, 84, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Hur, S.J.; Yang, H.S.; Moon, S.H.; Hwang, Y.H.; Park, G.B.; Joo, S.T. Discoloration characteristics of 3 major muscles from cattle during cold storage. J. Food Sci. 2009, 74, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Karamucki, T.; Jakubowska, M.; Rybarczyk, A.; Gardzielewska, J. The influence of myoglobin on the colour of minced pork loin. Meat Sci. 2013, 94, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Nerin, C.L.; Tovar, D.; Djenane, J. Stabilization of beef meat by a new active packaging containing natural antioxidants. J. Agric. Food Chem. 2006, 52, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat intake and risk of colorectal adenomas: A systematic review and meta-analysis of epidemiological studies. Cancer Causes Control 2013, 24, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.H.; Sabharanjak, S.M.; Zengin, G.; Mollica, A.; Szostak, A.; Simirgiotis, M.; Huminiecki, Ł.; Horbańczuk, O.H.; Nabavi, S.M.; Moca, A. Pecan nuts: A review of reported bioactivities and health effects. Trends Food Sci. Technol. 2018, 71, 246–257. [Google Scholar] [CrossRef]
- Duran-Montgé, P.; Realini, C.E.; Barroeta, A.C.; Lizardo, R.G.; Esteve-Garcia, E. De novo fatty acid synthesis and balance of fatty acids of pigs fed different fat sources. Livest. Sci. 2010, 132, 157–164. [Google Scholar] [CrossRef]
- Poławska, E.; Marchewka, J.; Cooper, R.G.; Sartowska, K.; Pomianowski, J.; Jóźwik, A.; Strzałkowska, N.; Horbańczuk, J.O. The ostrich meat—An updated review. II.—Nutritive value. Anim. Sci. Pap. Rep. 2011, 29, 89–97. [Google Scholar]
- Poławska, E.; Horbańczuk, J.O.; Pierzchała, M.; Strzałkowska, N.; Jóźwik, A.; Wójcik, A.; Pomianowski, J.; Gutkowska, K.; Wierzbicka, A.; Hoffman, L.C. Effect of dietary linseed and rapeseed supplementation on fatty acid profiles in the ostrich. Part 1. Muscles. Anim. Sci. Pap. Rep. 2013, 31, 239–248. [Google Scholar]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S.; Herbers, C.A.Z. Effect of packaging and physical characteristics of ground pork in long-term frozen storage. J. Food Sci. 1991, 56, 363–366. [Google Scholar] [CrossRef]
- Brewer, M.S.; Ikins, W.G.; Harbers, C.A.Z. TBA values, sensory characteristics and volatiles in ground pork during long-term frozen storage: Effects of packaging. J. Food Sci. 1992, 57, 558–563. [Google Scholar] [CrossRef]
- Juncher, D.; Ronn, B.; Mortensen, E.T.; Henckel, P.; Karlssonm, A.; Skibsted, L.H.; Bertelsen, G. Effect of pre-slaughter physiological conditions on the oxidative stability of colour and lipid during chill storage of pork. Meat Sci. 2001, 58, 347–357. [Google Scholar] [CrossRef]
- Horbańczuk, J.; Sales, J.; Celeda, T.; Konecka, A.; Zięba, G.; Kawka, P. Cholesterol Content and Fatty Acid Composition of Ostrich Meat as Influence by Subspecies. Meat Sci. 1998, 50, 385–388. [Google Scholar] [CrossRef]
- Horbańczuk, J.O.; Poławska, E.; Wójcik, A.; Hoffman, L.C. Influence of Frozen Storage on the Fatty Acid Composition of Ostrich Linseed and Rapeseed. S. Afr. J. Anim. Sci. 2015, 45, 129–136. [Google Scholar] [CrossRef]
- Horbańczuk, O.K.; Wierzbicka, A. Technological and nutritional properties of ostrich, emu and rhea meat quality. J. Vet. Res. 2016, 60, 279–286. [Google Scholar] [CrossRef]
- Horbańczuk, O.K.; Wierzbicka, A. Effects of Packaging Solutions on Shelf-Life of Ratite Meats. J. Vet. Res. 2017, 61, 279–285. [Google Scholar] [CrossRef]
- Peeters, E.; Driessen, B.; Geers, R. Influence of supplemental magnesium, tryptophan, vitamin C, vitamin E, and herbs on stress responses and pork quality. J. Anim. Sci. 2006, 84, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Pacetti, D.; Lucci, P.; Di Lecce, G.; Frega, N.G. Supplementation with high-oleic sunflower oil and α-tocopheryl acetate: Effects on meat pork lipids. Eur. J. Lipid Sci. Technol. 2008, 110, 381–391. [Google Scholar] [CrossRef]
- Godziszewska, J.; Guzek, D.; Głąbska, D.; Jóźwik, A.; Brodowska, M.; Głąbski, K.; Zarodkiewicz, M.; Gantner, M.; Wierzbicka, A. Nutrient oxidation in pork loin is influenced by feed supplementation and packing methods. J. Food Compos. Anal. 2016, 56, 18–24. [Google Scholar] [CrossRef]
- Jasińska, K.; Kurek, A. The effect of oil plants supplementation in pig diet on quality and nutritive value of pork meat. Anim. Sci. Pap. Rep. 2017, 35, 137–146. [Google Scholar]
- Sales, J.; Horbańczuk, J.O. Ratite Meat. World Poult. Sci. J. 1998, 54, 59–67. [Google Scholar] [CrossRef]
- Poławska, E.; Zdanowska-Sąsiadek, Ż.; Horbańczuk, J.; Pomianowski, J.F.; Jóźwik, A.; Tolik, D.; Raes, K.; De Smet, S. Effect of dietary organic and inorganic selenium supplementation on chemical, mineral and fatty acid composition of ostrich meat. CYTA J. Food 2016, 14, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Pogorzelska-Nowicka, E.; Atanasov, A.G.; Horbańczuk, J.; Wierzbicka, A. Bioactive Compounds in Functional Meat Products. Molecules 2018, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Głąbska, D.; Brodowska, M.; Sun, D.W.; Wierzbicka, A. Volotile compounds and fatty acids profile in Longossimus Dorsi muscle from pigs fed with feed containing bioactive components. LWT Food Sci. Technol. 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Brodowska, M.; Guzek, D.; Kołota, A.; Głąbska, D.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Wierzbicka, A. Effect of diet on oxidation and profile of volatile compounds of pork after freezing storage. J. Food Nutr. Res. 2016, 55, 40–47. [Google Scholar]
- Corino, C.; Musella, M.; Mourot, J. Influence of extruded linseed on growth, carcass composition and meat quality of slaughtered pigs at 110 and 160 kilograms of liveweight. J. Anim. Sci. 2008, 86, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Haak, L.; De Smet, S.; Fremaut, D.; Van Walleghem, K.; Raes, K. Fatty acid profile and oxidative stability of pork as influenced by duration and time of dietary linseed or fish oil supplementation. J. Anim. Sci. 2008, 86, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine Longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.; Dugan, M.E.R.; Larsen, I.L.; Thacker, R.; Rolland, D.C.; Aalhus, J.L. Oxidative stability in grinds from omega-3 enhanced pork. Can. J. Anim. Sci. 2011, 91, 623–634. [Google Scholar] [CrossRef]
- Sobotka, W.; Flis, M.; Antoszkiewicz, Z.; Lipiński, K.; Zduńczyk, Z. Effect of by-product antioxidants and vitamin E on the oxidative stability of pork from pigs fed diets supplemented with linseed oil. Arch. Tierernahr. 2012, 66, 27–38. [Google Scholar] [CrossRef]
- National Pork Producers Council (NPPC). Pork Quality Standards; National Pork Producers Council: Des Moines, IA, USA, 1999. [Google Scholar]
- Juárez, M.; Dugan, M.E.R.; Aldai, N.; Aalhus, J.L.; Patience, J.F.; Zijlstra, R.T.; Beaulieu, A.D. Increasing omega-3 levels through dietary co-extruded flaxseed supplementation negatively affects pork palatability. Food Chem. 2011, 126, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Okrouhlá, M.; Stupka, R.; Čítek, J.; Šprysl, M.; Brzobohatý, L.; Kluzáková, E. The effect of addition of rapeseed meal on the quality of pork meat and fat. Res. Pig Breed. 2012, 6, 52–54. [Google Scholar] [CrossRef]
- James, B.W.; Goodband, R.D.; Unruh, J.A.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S.; O’Quinn, P.R.; Andrews, B.S. Effect of creatine monohydrate on finishing pig growth performance, carcass characteristics and meat quality. Anim. Sci. Feed Technol. 2002, 96, 135–145. [Google Scholar] [CrossRef]
- Garry, D.J.; Mammen, P.P. Molecular insights into the functional role of myoglobin. Adv. Exp. Med. Biol. 2007, 618, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Psomiadou, E.; Tsimidou, M.; Hopia, A.; Kefalas, P.; Heinonen, K.W.M. Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur. Food Res. Technol. 2002, 214, 294–298. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 69–186. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A.; Mancini, R.A.; Seyfert, M.; Hunt, M.C. Interactions between Mitochondrial Lipid Oxidation and Oxymyoglobin Oxidation and the Effects of Vitamin E. J. Agric. Food Chem. 2005, 53, 6073–6079. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Phillips, A.L.; Liebler, D.C.; Faustman, C. Porcine oxymyoglobin and lipid oxidation in vitro. Meat Sci. 2003, 63, 767–775. [Google Scholar] [CrossRef]
- O’Grady, M.N.; Monahan, F.J.; Fallon, R.J.; Allen, P. Effects of dietary supplementation with vitamin E and organic selenium on the oxidative stability of beef. J. Anim. Feed Sci. 2001, 79, 2827–2834. [Google Scholar] [CrossRef]
- Suman, S.P.; Faustman, C.; Stamer, S.L.; Liebler, D.C. Proteomics of lipid oxidation-induced oxidation in porcine and bovine oxymyoglobins. Proteomics 2007, 7, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, G.; Lundström, K.; Tornberg, E. Contribution of pigment content, myoglobin forms and internal reflactance to the colour of pork loin and ham from pure breed pigs. Meat Sci. 2001, 59, 141–151. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Res. Int. 2003, 36, 787–795. [Google Scholar] [CrossRef]
- American Meat Science Association (AMSA). Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki Revisited: Equations for Spectrophotometric determination of myoglobin redox forms in aqueous meat extracts. J. Food Sci. 2004, 69, 717–720. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Statistical Approaches | I | II | III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Statistical Groups | Control | Supplemented Groups | Control Oil | RO | LO | VE | Se | VE+ | |
| Experimental Groups | |||||||||
| C (basal diet—BS) | x | ||||||||
| RO (BS + rapeseed oil) | x | x | |||||||
| R1 (BS + rapeseed oil + VE) | x | x | x | ||||||
| R2 (BS + rapeseed oil + Se) | x | x | x | ||||||
| R3 (BS + rapeseed oil + VE + Se) | x | x | x | ||||||
| LO (BS + linseed oil) | x | x | |||||||
| L1 (BS + linseed oil + VE) | x | x | x | ||||||
| L2 (BS + linseed oil + Se) | x | x | x | ||||||
| L3 (BS + linseed oil + VE + Se) | x | x | x | ||||||
| Trait | Control° (n = 6, N = 10) | Supplemented Groups□ (n = 36, N = 10) | Control Oil∆ (n = 12, N = 10) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min-Max) | Mean ± SD | Median (Min–Max) | |
| L* | 45.55 ± 4.52a | 45.33 (36.82–56.44) | 44.95 ± 4.77a | 44.50 (35.15–60.94) | 43.34 ± 3.56b | 43.62 (34.35–54.83) |
| a* | 11.87 ± 3.44a | 11.59 (6.95–19.59) | 11.93 ± 4.30a | 11.33 (3.02–26.52) | 14.50 ± 3.5b | 14.29 (5.61–22.46) |
| b* | 4.53 ± 1.70ab | 4.4 (1.49–8.92) | 4.21 ± 1.87a | 3.84 (0.35–12.53) | 5.08 ± 1.99b | 4.75 (1.4–11.47) |
| C* | 12.82 ± 3.41a | 12.44 (7.49–21.48) | 12.73 ± 4.42a | 12.11 (4.04–29.33) | 15.47 ± 3.61b | 15.27 (6.54–25.21) |
| h° | 0.37 ± 0.14 | 0.35 (0.15–0.77) | 0.35 ± 0.13 | 0.32 (0.07–1.04) | 0.34 ± 0.12 | 0.33 (0.12–0.67) |
| Trait | RO■ (n = 6, N = 10) | LO▲ (n = 6, N = 10) | ||
|---|---|---|---|---|
| Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | |
| L* | 43.92 ± 2.95 | 44.01 (36.57–54,83) | 42,75 ± 4,02 * | 42.82 (34.35–51.78) |
| a* | 15.24 ± 2.86a * | 15.09 (9.9–22.5) | 13.77 ± .93b * | 13.8 (5.61–21.3) |
| b* | 5.32 ± 1.84 | 4.75 (2.43–11.5) | 4.85 ± 2.11 | 4.71 (1.4–9.23) |
| C* | 16.21 ± 3.02a * | 16.27 (11.29–25.22) | 14.73 ± 4.0b * | 14.81 (6.54–23.20) |
| h° | 0.34 ± 0.09 | 0.32 (0.18–0.6) | 0.35 ± 0.14 | 0.34 (0.12–0.67) |
| Trait | VESe◊ (n = 12, N = 10) | Se+ (n = 12, N = 10) | VEˣ (n = 12, N = 10) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (Min–Max) | Mean ± SD | Median (min-max) | Mean ± SD | Median (Min–Max) | |
| L* | 46.66 ± 4.4a | 45.32 (36.66–60.94) | 43.83 ± 4.98b | 43.38 (35.15–58.62) | 45 ± 4.74a | 44.75 (35.60–57.26) |
| a* | 11.95 ± 4.22ab | 11.41 (3.02–24.91) | 11.83 ± 4.22a | 12.56 (5.61–26.52) | 11.01 ± 4.31b | 10.57 (3.91–24.89) |
| b* | 4.29 ± 1.81a | 3.88 (1.14–9.73) | 4.72 ± 2.06a | 4.30 (1.75–12.53) | 3.61 ± 1.57b* | 3.38 (0.33–8.66) |
| C* | 12.8 ± 4.28ab | 12.32 (5.5–25.68) | 13.78 ± 4.36a | 13.29 (5.88–29.33) | 11.66 ± 4.41b | 11.19 (4.04–26.35) |
| h° | 0.36 ± 0.14 | 0.33 (0.103–1.035) | 0.36 ± 0.13 | 0.33 (0.159–0.69) | 0.33 ± 0.12 | 0.32 (0.07–0.68) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorzelska-Nowicka, E.; Godziszewska, J.; Horbańczuk, J.O.; Atanasov, A.G.; Wierzbicka, A. The Effect of PUFA-Rich Plant Oils and Bioactive Compounds Supplementation in Pig Diet on Color Parameters and Myoglobin Status in Long-Frozen Pork Meat. Molecules 2018, 23, 1005. https://doi.org/10.3390/molecules23051005
Pogorzelska-Nowicka E, Godziszewska J, Horbańczuk JO, Atanasov AG, Wierzbicka A. The Effect of PUFA-Rich Plant Oils and Bioactive Compounds Supplementation in Pig Diet on Color Parameters and Myoglobin Status in Long-Frozen Pork Meat. Molecules. 2018; 23(5):1005. https://doi.org/10.3390/molecules23051005
Chicago/Turabian StylePogorzelska-Nowicka, Ewelina, Jolanta Godziszewska, Jarosław O. Horbańczuk, Atanas G. Atanasov, and Agnieszka Wierzbicka. 2018. "The Effect of PUFA-Rich Plant Oils and Bioactive Compounds Supplementation in Pig Diet on Color Parameters and Myoglobin Status in Long-Frozen Pork Meat" Molecules 23, no. 5: 1005. https://doi.org/10.3390/molecules23051005





