Solid-Phase Synthesis of Azole-Comprising Peptidomimetics and Coordination of a Designed Analog to Zn2+
Abstract
:1. Introduction
2. Results
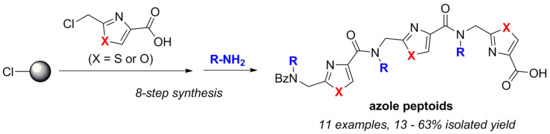
2.1. Synthesis of Trimeric Azole Peptoids
2.2. Metal Coordination of 9 in Aqueous Buffer
3. Discussion
4. Materials and Methods
4.1. General Experimental Information
4.2. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
4.3. General Procedure for Synthesis of N-Benzoylated Azole Peptoid Trimers
4.4. Data for Azole Peptoids Prepared
4.4.1. Thiazole Peptoid Trimer 2a
4.4.2. Oxazole Peptoid Trimer 2b
4.4.3. Thiazole Peptoid Trimer 3a
4.4.4. Oxazole Peptoid Trimer 3b
4.4.5. Thiazole Peptoid Trimer 4a
4.4.6. Oxazole Peptoid Trimer 4b
4.4.7. Thiazole Peptoid Trimer 5
4.4.8. Thiazole Peptoid Trimer 6
4.4.9. Thiazole Peptoid Trimer 7
4.4.10. Thiazole Peptoid Trimer 8
4.4.11. Thiazole Peptoid Trimer 9
4.5. Evaluation of Zn2+ Binding by 9
4.5.1. Preparation of a Stock Solution of 9
4.5.2. Zn2+ Titrations to 9 Monitored by UV-Vis Spectroscopy
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Knight, A.S.; Kulkarni, R.U.; Zhou, E.Y.; Franke, J.M.; Miller, E.W.; Francis, M.B. A modular platform to develop peptoid-based selective fluorescent metal sensors. Chem. Commun. 2017, 53, 3477–3480. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G. Conformational control in metallofoldamers: Design, synthesis and structural properties. Eur. J. Org. Chem. 2009, 2009, 5699–5710. [Google Scholar] [CrossRef]
- Prathap, K.J.; Maayan, G. Metallopeptoids as efficient biomimetic catalysts. Chem. Commun. 2015, 51, 11096–11099. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G.; Ward, M.D.; Kirshenbaum, K. Folded biomimetic oligomers for enantioselective catalysis. Proc. Natl. Acad. Sci. USA 2009, 106, 13679–13684. [Google Scholar] [CrossRef] [PubMed]
- Gellman, S.H. Foldamers: A manifesto. Acc. Chem. Res. 1998, 31, 173–180. [Google Scholar] [CrossRef]
- Hill, D.J.; Mio, M.J.; Prince, R.B.; Hughes, T.S.; Moore, J.S. A field guide to foldamers. Chem. Rev. 2001, 101, 3893–4011. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.A.; Blackwell, H.E. Structure–function relationships in peptoids: Recent advances toward deciphering the structural requirements for biological function. Org. Biomol. Chem. 2009, 7, 1508. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; Choi, S.; Shandler, S.; DeGrado, W.F. Foldamers as versatile frameworks for the design and evolution of function. Nat. Chem. Biol. 2007, 3, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G.; Yoo, B.; Kirshenbaum, K. Heterocyclic amines for the construction of peptoid oligomers bearing multi-dentate ligands. Tetrahedron Lett. 2008, 49, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Baskin, M.; Maayan, G. A rationally designed metal-binding helical peptoid for selective recognition processes. Chem. Sci. 2016, 7, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Bertram, A.; Pattenden, G. Marine metabolites: Metal binding and metal complexes of azole-based cyclic peptides of marine origin. Nat. Prod. Rep. 2007, 24, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Van den Brenk, A.L.; Byriel, K.A.; Fairlie, D.P.; Gahan, L.R.; Hanson, G.R.; Hawkins, C.J.; Jones, A.; Kennard, C.H.L.; Moubaraki, B.; Murray, K.S. Crystal structure and electrospray ionization mass spectrometry, electron paramagnetic resonance, and magnetic susceptibility study of [Cu2(ascidH2)(1,2-μ-CO3)(H2O)2]·2H2O, the bis(copper(II)) complex of ascidiacyclamide (ascidH4), a cyclic peptide isolated from the ascidian Lissoclinum patella. Inorg. Chem. 1994, 33, 3549–3557. [Google Scholar] [CrossRef]
- Wipf, P.; Venkatraman, S.; Miller, C.P.; Geib, S.J. Metal complexes of marine peptide metabolites: A novel Ag4 Cluster. Angew. Chem. Int. Ed. Engl. 1994, 33, 1516–1518. [Google Scholar] [CrossRef]
- Wipf, P. Synthetic studies of biologically active marine cyclopeptides. Chem. Rev. 1995, 95, 2115–2134. [Google Scholar] [CrossRef]
- Jolliffe, K.A. Backbone-modified cyclic peptides: New scaffolds for supramolecular chemistry. Supramol. Chem. 2005, 17, 81–86. [Google Scholar] [CrossRef]
- Dudin, L.; Pattenden, G.; Viljoen, M.S.; Wilson, C. Synthesis of novel N-methylated thiazole-based cyclic octa- and dodecapeptides. Tetrahedron 2005, 61, 1257–1267. [Google Scholar] [CrossRef]
- Simon, R.J.; Kania, R.S.; Zuckermann, R.N.; Huebner, V.D.; Jewell, D.A.; Banville, S.; Ng, S.; Wang, L.; Rosenberg, S.; Marlowe, C.K. Peptoids: A modular approach to drug discovery. Proc. Natl. Acad. Sci. USA 1992, 89, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N. Peptoid origins. Biopolymers 2011, 96, 545–555. [Google Scholar] [CrossRef]
- Baskin, M.; Panz, L.; Maayan, G. Versatile ruthenium complexes based on 2,2′-bipyridine modified peptoids. Chem. Commun. 2016, 52, 10350–10353. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G.; Ward, M.D.; Kirshenbaum, K. Metallopeptoids. Chem. Commun. 2009, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.S.; Zhou, E.Y.; Francis, M.B. Development of peptoid-based ligands for the removal of cadmium from biological media. Chem. Sci. 2015, 6, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.S.; Zhou, E.Y.; Pelton, J.G.; Francis, M.B. Selective chromium(VI) ligands identified using combinatorial peptoid libraries. J. Am. Chem. Soc. 2013, 135, 17488–17493. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Chu, T.K.; Dill, K.A.; Zuckermann, R.N. Biomimetic nanostructures: Creating a high-affinity zinc-binding site in a folded nonbiological polymer. J. Am. Chem. Soc. 2008, 130, 8847–8855. [Google Scholar] [CrossRef] [PubMed]
- Aditya, A.; Kodadek, T. Incorporation of heterocycles into the backbone of peptoids to generate diverse peptoid-inspired one bead one compound libraries. ACS Comb. Sci. 2012, 14, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Hjelmgaard, T.; Faure, S.; Staerk, D.; Taillefumier, C.; Nielsen, J. Efficient and versatile COMU-mediated solid-phase submonomer synthesis of arylopeptoids (oligomeric N-substituted aminomethyl benzamides). Org. Biomol. Chem. 2011, 9, 6832–6843. [Google Scholar] [CrossRef] [PubMed]
- Burkoth, T.S.; Fafarman, A.T.; Charych, D.H.; Connolly, M.D.; Zuckermann, R.N. Incorporation of unprotected heterocyclic side chains into peptoid oligomers via solid-phase submonomer synthesis. J. Am. Chem. Soc. 2003, 125, 8841–8845. [Google Scholar] [CrossRef] [PubMed]
- Figliozzi, G.M.; Goldsmith, R.; Ng, S.C.; Banville, S.C.; Zuckermann, R.N. [25] Synthesis of N-substituted glycine peptoid libraries. Methods Enzymol. 1996, 267, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Dobrawa, R.; Lysetska, M.; Ballester, P.; Grüne, M.; Würthner, F. Fluorescent supramolecular polymers: Metal directed self-assembly of perylene bisimide building blocks. Macromolecules 2005, 38, 1315–1325. [Google Scholar] [CrossRef]
- Nakamoto, K. Ultraviolet spectra and structures of 2,2′-bipyridine and 2,2′,2′′-terpyridine in aqueous solution. J. Phys. Chem. 1960, 64, 1420–1425. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds detailed here are available from the authors. |



| Compound | Structure | Crude Yield | Crude Purity 1 | Isolated Yield 4 |
|---|---|---|---|---|
| 2a (X = S) |  | 2a: 79% | 2a: 85% | 2a: 61% |
| 2b (X = O) | 2b: 60% | 2b: 60% | 2b: 33% | |
| 3a (X = S) |  | 3a: 58% | 3a: 81% | 3a: 13% |
| 3b (X = O) | 3b: 79% | 3b: 77% | 3b: 31% | |
| 4a (X = S) |  | 4a: 80% | 4a: 77% | 4a: 51% |
| 4b (X = O) | 4b: 63% | 4b: 84% | 4b: 48% | |
| 5 |  | 65% | 89% | 40% |
| 6 |  | 52% | 80% | 15% |
| 7 |  | 110% 2 | 98% | 63% |
| 8 |  | ND 3 | ND 3 | 39% |
| 9 |  | 67% | ND 3 | 29% |
| 10 |  | -- 5 | -- | -- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, A.; Koh, A.H.M.; Gate, G.; Calkins, A.L.; McComas, K.N.; Fuller, A.A. Solid-Phase Synthesis of Azole-Comprising Peptidomimetics and Coordination of a Designed Analog to Zn2+. Molecules 2018, 23, 1035. https://doi.org/10.3390/molecules23051035
Mohan A, Koh AHM, Gate G, Calkins AL, McComas KN, Fuller AA. Solid-Phase Synthesis of Azole-Comprising Peptidomimetics and Coordination of a Designed Analog to Zn2+. Molecules. 2018; 23(5):1035. https://doi.org/10.3390/molecules23051035
Chicago/Turabian StyleMohan, Aanchal, Allyson H. M. Koh, Gregory Gate, Anna L. Calkins, Kyra N. McComas, and Amelia A. Fuller. 2018. "Solid-Phase Synthesis of Azole-Comprising Peptidomimetics and Coordination of a Designed Analog to Zn2+" Molecules 23, no. 5: 1035. https://doi.org/10.3390/molecules23051035