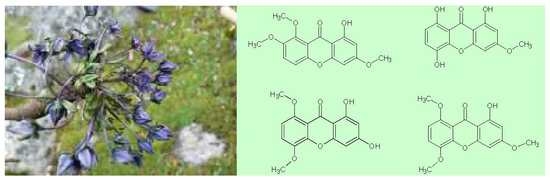
Xanthones Content in Swertia multicaulis D. Don from Nepal
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Materials and Extraction
3.2. HPLC and LC–MS Measurements
3.3. LC–NMR Measurement and Preparation of Xanthones
3.4. Determination of Xanthones Using NMR
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brahmachari, G.; Mondal, S.; Gangopadhyay, A.; Gorai, D.; Mukhopadhyay, B.; Saha, S.; Arun, K.; Brahmachari, A.K. Swertia (Gentianaceae): Chemical and pharmacological aspects. Chem. Biodivers. 2004, 1, 1627–1651. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaveri, J.; Cárdenas-Rodriguez, N.; Orozco-Ibarra, M.; Peréz-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, C.; Tang, S.; Xiao, H.; Kakiuchi, N.; Kida, H.; Hattori, M. Qualitative and quantitative analysis of Swertia herbs by high performance liquid chromatography-diode array detector-mass spectrometry (HPLC-DAD-MS). Chem. Pharm. Bull. 2008, 56, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Shekarchi, M.; Hajimehdipoor, H.; Khanavi, M.; Adib, N.; Bozorgi, M.; Akbari-Adergani, B. A validated method for analysis of Swerchirin in Swertia longifolia Boiss. by high performance liquid chromatography. Pharmacogn. Mag. 2010, 6, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-G.; Wang, W.; Zhang, Q.-Y.; Cheng, J.; Avula, B.; Khan, I.A.; Guo, D.-A. Identification of xanthones from Swertia punicea using high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2913–2923. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G. Naturally occurring xanthones: Chemistry and biology. J. Appl. Chem. 2013, 621459. [Google Scholar] [CrossRef]
- Negi, J.S.; Singh, P.; Joshi, G.; Rawat, M.S.M. RP-HPLC analysis and antidiabetic activity of Swertia paniculata. Nat. Prod. Commun. 2010, 5, 907–910. [Google Scholar] [PubMed]
- Luo, C.-T.; Mao, S.-S.; Liu, F.-L.; Yang, M.-X.; Chen, H.; Kurihara, H.; Li, Y. Antioxidant xanthones from Swertia mussotii, a high altitude plant. Fitoterapia 2013, 91, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shi, G.; Chen, X.; Chen, F.; Yao, R.; Wang, Z. Loading of free radicals on the functional graphene combined with liquid chromatography-tandem mass spectrometry screening method for the detection of radical-scavening natural antioxidants. Anal. Chim. Acta 2013, 802, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.-L.; Rodriguez, S.; Hostettmann, K.; Hiller, W. Liquid chromatography/ultra violet/mass spectrometric and liquid chromatography/nuclear magnetic resonance spectroscopic analysis of crude extracts of Gentianaceae species. Phytochem. Anal. 1997, 8, 97–104. [Google Scholar] [CrossRef]
- Chintalwar, G.J.; Chattapadhyay, S. Structural confirmation of decussatin, a Swertia decussata xanthone. Nat. Prod. Res. 2006, 20, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Chen, T.; Wang, P.; Chen, G.; You, J.; Liu, Y.; Li, Y. Preparative separation of methylswertianin, swerchirin and decussatin from the Tibetan medicinal plant Swertia mussotii using high-speed counter-current chromatography. Phytochem. Anal. 2012, 23, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Shakya, N.; Thapa, K.; Pant, D.R. Phytochemical investigation of crude methanol extracts of different species of Swertia from Nepal. BMC Res. Notes 2015, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Jain, D.C.; Bhakuni, R.S. Phytochemicals from genus Swertia and their biological activities. Indian J. Chem. Sect. B 2000, 39, 565–586. [Google Scholar]
- Jamwal, A. Systematic review on xanthones and others isolates from genus Swertia. IJPCBS 2012, 1, 1464–1482. [Google Scholar]
- IUCN Nepal. National Register of Medicinal Plants; IUCN: Kathmandu, Nepal, 2000; 163p. [Google Scholar]
- Manandhar, N.P. Plants and People of Nepal; Timber Press: Portland, OR, USA, 2002; 599p. [Google Scholar]
- Baral, S.R.; Kurmi, P.P. A Compendium of Medicinal Plants in Nepal; Rachana Sharma: Kathmandu, Nepal, 2006; 534p. [Google Scholar]
- Joshi, K. Swertia L. (Gentianaceae) in Nepal: Ethnobotany and agenda for sustainable management. Ethnobot. Leaflets 2008, 12, 1–6. [Google Scholar]
- Joshi, K. Indigenous uses of Swertia (Gentianaceae) in Nepal—Present status and agenda for sustainable management. J. Nat. Prod. Plant Resour. 2011, 1, 137–141. [Google Scholar]
- Uprety, Y.; Asselin, H.; Boon, E.K.; Yadav, S.; Shrestha, K.K. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa district, central Nepal. J. Ethnobiol. Ethnomed. 2010, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Khetwal, K.S.; Joshi, B.; Bisht, R.S. Tri- and tetraoxygenated xanthones from Swertia petiolata. Phytochemistry 1990, 29, 1265–1267. [Google Scholar] [CrossRef]
- Kanamori, H.; Sakamoto, I.; Mizuta, M.; Hashimoto, K.; Tanaka, O. Studies on the mutagenicity of Swertiae herba. I. Identification of the mutagenic components. Chem. Pharm. Bull. 1984, 32, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Hiroshi, S.; Motoyoshi, S.; Yuji, M.; Yuki, H.; Shizume, T.; Kouchiro, S. Amarogentin, Amaroswerin and four xanthones from hairy root cultures of Swertia japonica. Phytochemistry 1990, 29, 1563–1565. [Google Scholar] [CrossRef]
- Rokaya, M.B. Diversity, Distribution and Conservation of Medicinal Plants in Nepal. Ph.D. Thesis, Charles University, Prague, Czech Republic, 2011. Available online: https://is.cuni.cz/webapps/zzp/detail/84672/?lang=en (accessed on 30 April 2018).
- Ho, T.-N.; Pringle, J.S. Gentianaceae. In Flora in China; Wu, Z.Y., Raven, P.H., Eds.; Missouri Botanical Garden: St. Louis, MO, USA, 1995; 139p. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| LC/MS APCI | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Peak 7 | Peak 8 |
|---|---|---|---|---|---|---|---|---|
| [M + H]+ | 289 | 261 | 289 | 303 | 303 | 275 | 303 | 273 |
| Sample | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Peak 7 | Peak 8 |
|---|---|---|---|---|---|---|---|---|
| SM1 | 1134 | 5337 | 1472 | 1302 | 4643 | 316 | 1957 | 167 |
| SM2 | 406 | 1295 | 1167 | 1194 | 2996 | 224 | 3230 | 146 |
| SM3 | 913 | 2600 | 843 | 859 | 2672 | 260 | 2230 | 185 |
| SM4 | 811 | 1934 | 1718 | 1556 | 3144 | 423 | 2683 | 224 |
| Average | 816 | 2791 | 1300 | 1228 | 3364 | 306 | 2525 | 181 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timsina, B.; Kindlmann, P.; Rokaya, M.B.; Vrchotová, N.; Tříska, J.; Horník, Š.; Sýkora, J. Xanthones Content in Swertia multicaulis D. Don from Nepal. Molecules 2018, 23, 1067. https://doi.org/10.3390/molecules23051067
Timsina B, Kindlmann P, Rokaya MB, Vrchotová N, Tříska J, Horník Š, Sýkora J. Xanthones Content in Swertia multicaulis D. Don from Nepal. Molecules. 2018; 23(5):1067. https://doi.org/10.3390/molecules23051067
Chicago/Turabian StyleTimsina, Binu, Pavel Kindlmann, Maan B. Rokaya, Naděžda Vrchotová, Jan Tříska, Štěpán Horník, and Jan Sýkora. 2018. "Xanthones Content in Swertia multicaulis D. Don from Nepal" Molecules 23, no. 5: 1067. https://doi.org/10.3390/molecules23051067