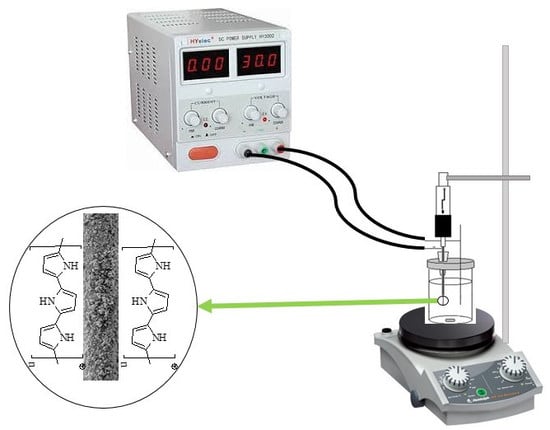
Comparison of the Conventional and Electroenhanced Direct-Immersion Solid-Phase Microextraction for Sampling of Nicotine in Biological Fluids of the Human Body
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of PPy Fiber
2.2. Effect of Sample pH
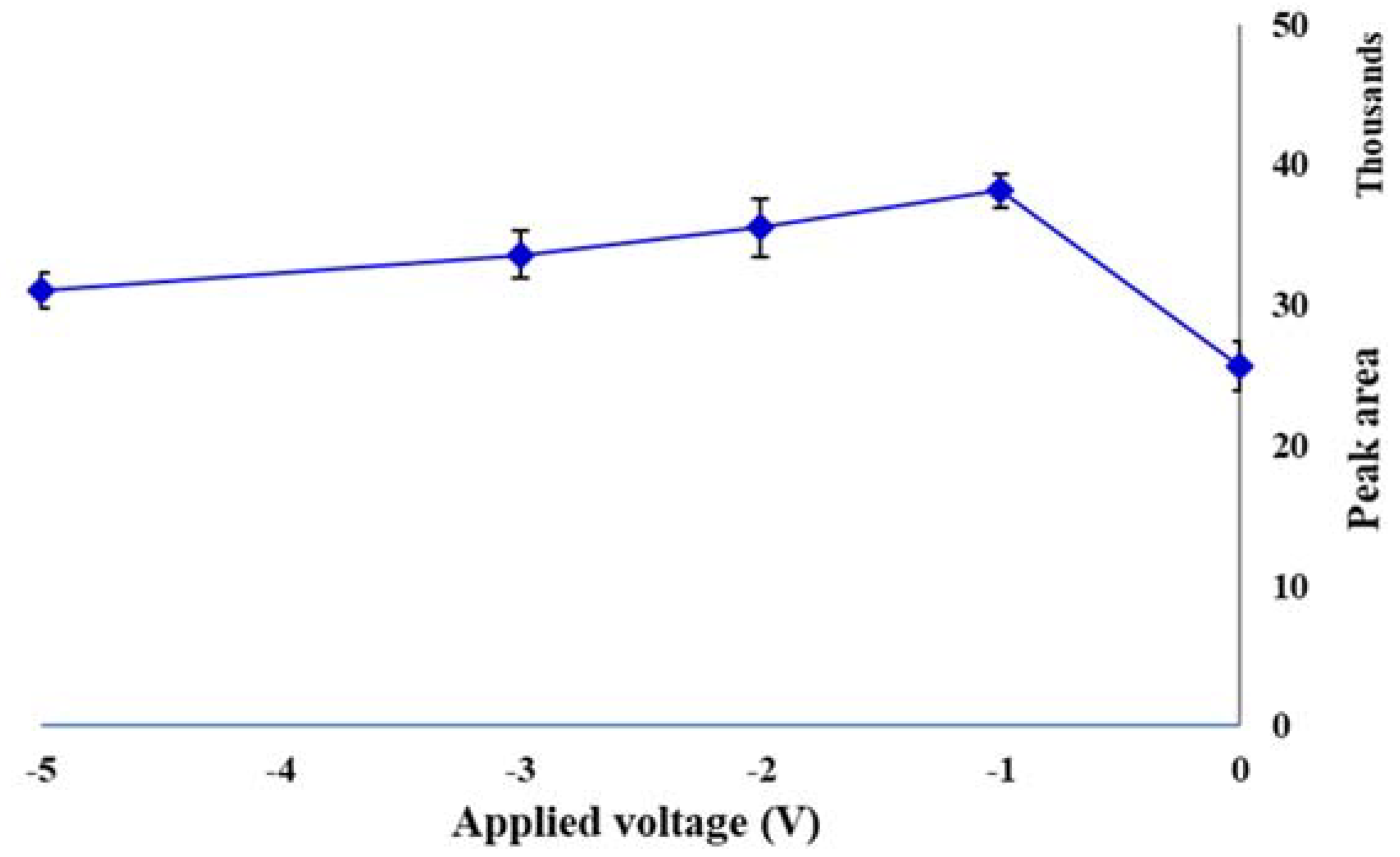
2.3. Applied Voltage
2.4. Study of Extraction Temperature and Time
2.5. Effect of Stirring Rate and Ionic Strength of the Sample Solution
2.6. Desorption Temperature and Time
2.7. Comparison of the PPy-Coated Fiber with Commercial Fibers
2.8. Method Validation
2.9. Analysis of Real Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Apparatus
3.3. Preparation of the Fiber Substrate and Its Coating Using the EPD Method
3.4. Extraction Procedure
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.R.; Hajipour, S.; Heidari, N. Cooling-assisted microextraction: Comparison of techniques and applications. Trends Anal. Chem. 2016, 77, 54–65. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Vuckovic, D.; de Lannoy, I.; Gien, B.; Shirey, R.E.; Sidisky, L.M.; Dutta, S.; Pawliszyn, J. In vivo solid-phase microextraction: Capturing the elusive portion of metabolome. Angew. Chem. Int. Ed. 2011, 50, 5344–5348. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.; Dowlatshah, S.; Nouraei, N.; Heidari, N.; Yazdankhah, F. A solid-phase microextraction platinized stainless steel fiber coated with a multiwalled carbon nanotube-polyaniline nanocomposite film for the extraction of thymol and carvacrol in medicinal plants and honey. J. Chromatogr. A 2015, 1406, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.; Shadabi, S.; Hajipour, S.; Nasirian, A.; Borzouei, M.; Hassani-Moghadam, E.; Hashemi, P. Comparison of ultrasound-assisted headspace solid-phase microextraction and hydrodistillation for the identification of major constituents in two species of hypericum. J. Chromatogr. Sci. 2015, 54, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, E.; Souza-Silva, É.A.; Pawliszyn, J. Headspace versus direct immersion solid phase microextraction in complex matrixes: Investigation of analyte behavior in multicomponent mixtures. Anal. Chem. 2015, 87, 8448–8456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pawliszyn, J. Headspace solid-phase microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Song, H.-N.; Kim, C.H.; Lee, W.-Y.; Cho, S.-H. Simultaneous determination of volatile organic compounds with a wide range of polarities in urine by headspace solid-phase microextraction coupled to gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, G.; Huang, S.; Qiu, J.; Jiang, R.; Zhu, F.; Ouyang, G. Application of in vivo solid-phase microextraction in environmental analysis. Trends Anal. Chem. 2016, 85, 26–35. [Google Scholar] [CrossRef]
- Ho, T.D.; Yu, H.; Cole, W.T.S.; Anderson, J.L. Ultraviolet photoinitiated on-fiber copolymerization of ionic liquid sorbent coatings for headspace and direct immersion solid-phase microextraction. Anal. Chem. 2012, 84, 9520–9528. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, J.; Song, X.; Wang, Y.; Ha, J.; Chen, X.; Wang, X. An electrochemically enhanced solid-phase microextraction approach based on a multi-walled carbon nanotubes/nafion composite coating. J. Chromatogr. A 2010, 1217, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Basheer, C.; Al-Arfaj, A.R. Application of electro-enhanced solid-phase microextraction for determination of phthalate esters and bisphenol a in blood and seawater samples. Talanta 2013, 115, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mullett, W.M.; Pawliszyn, J. Electrochemically controlled solid-phase microextraction based on conductive polypyrrole films. Anal. Chem. 2002, 74, 4855–4859. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Sanz, J.; Cámara, C. Recent developments in solid-phase microextraction coatings and related techniques. J. Chromatogr. A 2006, 1103, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, R.; Bai, S.; Wang, C.; Wang, Z. A solid phase microextraction fiber coated with graphene–poly (ethylene glycol) composite for the extraction of volatile aromatic compounds from water samples. Talanta 2014, 119, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Zanjani, M.O.; Mehdinia, A. A review on procedures for the preparation of coatings for solid phase microextraction. Microchim. Acta 2014, 181, 1169–1190. [Google Scholar] [CrossRef]
- Mohammadkhani, E.; Yamini, Y.; Rezazadeh, M.; Seidi, S. Electromembrane surrounded solid phase microextraction using electrochemically synthesized nanostructured polypyrrole fiber. J. Chromatogr. A 2016, 1443, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Abdolhosseini, S.; Ghiasvand, A.; Heidari, N. A high area, porous and resistant platinized stainless steel fiber coated by nanostructured polypyrrole for direct HS-SPME of nicotine in biological samples prior to GC-FID quantification. J. Chromatogr. B 2017, 1061–1062, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, C.; Wu, P.; Li, X.; Zhang, M.; Zhu, J. Polypyrrole capacitance characteristics with different doping ions and thicknesses. Phys. Chem. Chem. Phys. 2017, 19, 21165–21173. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhao, Y.; Liu, P.; Zhang, X.; Wang, S. Electrochemical properties and the determination of nicotine at a multi-walled carbon nanotubes modified glassy carbon electrode. Microchim. Acta 2010, 168, 31–36. [Google Scholar] [CrossRef]
- Miller, E.I.; Norris, H.-R.K.; Rollins, D.E.; Tiffany, S.T.; Wilkins, D.G. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. B 2010, 878, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Patel, D.K. Liquid-phase microextraction combined with gas chromatography mass spectrometry for rapid determination of nicotine in one-drop of nightshades vegetables and commercial food products. Food Chem. 2010, 122, 314–318. [Google Scholar] [CrossRef]
- Daneshfar, A.; Khezeli, T. Headspace solid phase microextraction of nicotine using thin layer chromatography plates modified with carbon dots. Microchim. Acta 2015, 182, 209–216. [Google Scholar] [CrossRef]
- Brunnemann, K.; Hoffmann, D. The pH of tobacco smoke. Food Cosmet. Toxicol. 1974, 12, 115–124. [Google Scholar] [CrossRef]
- Barlow, R.B.; Hamilton, J.T. Effects of pH on the activity of nicotine and nicotine monomethiodide on the rat diaphragm preparation. Br. J. Pharmacol. Chemother. 1962, 18, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, A.; Haddad, P.R.; Quirino, J.P. The electric field—An emerging driver in sample preparation. Trends Anal. Chem. 2016, 80, 604–611. [Google Scholar] [CrossRef]
- Chai, X.; He, Y.; Ying, D.; Jia, J.; Sun, T. Electrosorption-enhanced solid-phase microextraction using activated carbon fiber for determination of aniline in water. J. Chromatogr. A 2007, 1165, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent developments in solid-phase microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Toraño, J.S.; van Kan, H.J. Simultaneous determination of the tobacco smoke uptake parameters nicotine, cotinine and thiocyanate in urine, saliva and hair, using gas chromatography-mass spectrometry for characterisation of smoking status of recently exposed subjects. Analyst 2003, 128, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, E.C.; de Oliveira, D.M.; de Siqueira, M.E.P.B.; Arruda, M.A.Z. On-line molecularly imprinted solid-phase extraction for the selective spectrophotometric determination of nicotine in the urine of smokers. Anal. Chim. Acta 2009, 635, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhang, L.; Wu, J.; Fritz, J.W.; Medeiros, S.; Rego, C. Ultrasonic extraction and capillary gas chromatography determination of nicotine in pharmaceutical formulations. Anal. Chim. Acta 2004, 526, 35–39. [Google Scholar] [CrossRef]
- Aresta, A.; Palmisano, F.; Zambonin, C.G. Simultaneous determination of caffeine, theobromine, theophylline, paraxanthine and nicotine in human milk by liquid chromatography with diode array UV detection. Food Chem. 2005, 93, 177–181. [Google Scholar] [CrossRef]
- Millet, A.; Stintzing, F.; Merfort, I. Validation of a GC-FID method for rapid quantification of nicotine in fermented extracts prepared from nicotiana tabacum fresh leaves and studies of nicotine metabolites. J. Pharm. Biomed. Anal. 2009, 49, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Inoue, R.; Yagi, K.; Saito, K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography—Mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.R.; Nouriasl, K.; Yazdankhah, F. Comparison of the atmospheric- and reduced-pressure HS-SPME strategies for analysis of residual solvents in commercial antibiotics using a steel fiber coated with a multiwalled carbon nanotube/polyaniline nanocomposite. Anal. Bioanal. Chem. 2018, 410, 361–371. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Method | Sample | LDR (μg mL−1) | LOD (ng mL−1) | RSD (%) | Ref. |
|---|---|---|---|---|---|
| LE-PTC-GC-MS | Urine, saliva and hair | 0.01–3 | 0.60 | 3–20 | [30] |
| MISPE-UV | Spiked urine | 0.17–9.7 | NR | 10 | [31] |
| UE-GC-FID | Pharmaceutical formulations | 1–500 | 250 | 2 | [32] |
| LC-UVDAD | Human milk | NR | 13 | 5.8 | [33] |
| GC-FID | Aqueous extract of tabacum leaves | 5–60 | 500 | 3.2 | [34] |
| IT-SPME-LC-MS | Human urine and saliva | 0.0005–0.02 | 0.015–0.04 | 4.7–11.3 | [35] |
| DI-SPME-GC-FID | Urine and plasma | 0.1–10 | 10 | 6.1 | This work |
| EE-DI-SPME-GC-FID | Urine and plasma | 0.001–10 | 0.3 | 4.6 | This work |
| Sample | Added (µg mL−1) | DI-SPME-GC-FID | EE-SPME-GC-FID | ||
|---|---|---|---|---|---|
| µg mL−1 | R (%) | µg mL−1 | R (%) | ||
| Urine 1 (Male, 2 years) | 0 | NF | --- | NF | --- |
| 2 | 1.82 (8.6) | 90 | 2.20 (5.1) | 110 | |
| Urine 2 (Female, non-smoker, 23 years) | 0 | NF | --- | NF | --- |
| 2 | 1.88 (7.2) | 94 | 2.06 (4.9) | 103 | |
| Plasma 1 (Male, smoker, 47 years) | 0 | 1.43 (9.5) | --- | 1.95 (7.4) | --- |
| 2 | 3.57 (6.3) | 105 | 3.61 (3.8) | 91 | |
| Plasma 2 (Male, smoker, 38 years) | 0 | 2.1 (5.8) | --- | 2.47 (4.5) | --- |
| 2 | 3.03 (4.7) | 75 | 4.4 (3.6) | 98 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdolhosseini, S.; Ghiasvand, A.R.; Heidari, N. Comparison of the Conventional and Electroenhanced Direct-Immersion Solid-Phase Microextraction for Sampling of Nicotine in Biological Fluids of the Human Body. Molecules 2018, 23, 1171. https://doi.org/10.3390/molecules23051171
Abdolhosseini S, Ghiasvand AR, Heidari N. Comparison of the Conventional and Electroenhanced Direct-Immersion Solid-Phase Microextraction for Sampling of Nicotine in Biological Fluids of the Human Body. Molecules. 2018; 23(5):1171. https://doi.org/10.3390/molecules23051171
Chicago/Turabian StyleAbdolhosseini, Sana, Ali Reza Ghiasvand, and Nahid Heidari. 2018. "Comparison of the Conventional and Electroenhanced Direct-Immersion Solid-Phase Microextraction for Sampling of Nicotine in Biological Fluids of the Human Body" Molecules 23, no. 5: 1171. https://doi.org/10.3390/molecules23051171