Plasma Pharmacokinetic Determination of Canagliflozin and Its Metabolites in a Type 2 Diabetic Rat Model by UPLC-MS/MS
Abstract
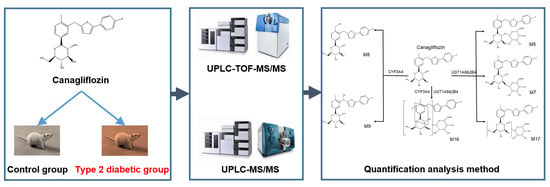
:1. Introduction
2. Results and Discussion
2.1. Identification of Canagliflozin and Its Metabolites by UPLC-TOF-MS/MS
2.2. UPLC-MS/MS Quantification Method
2.2.1. Optimization of UPLC-MS/MS Parameters
2.2.2. Linearity, Sensitivity and Specificity
2.2.3. Accuracy, Precision and Matrix Effect
2.2.4. Stability
2.3. Pharmacokinetics in Type 2Diabetic Rats
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. UPLC-TOF-MS/MS Conditions
3.4. UPLC-MS/MS Conditions
3.5. Preparation of Stock and Working Solutions, Calibration Standards and Quality Control Samples
3.6. Sample Extraction Procedure
3.7. Method Validation
3.8. STZ-Induced Type 2 Diabetic Rats
3.9. Application to Pharmacokinetic Study in Type 2 Diabetic Rats
3.10. Data Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012, 35 (Suppl. S1), S11–S63. [Google Scholar]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Hirayama, B.A.; Loo, D.F. Active sugar transport in health and disease. J. Intern. Med. 2007, 261, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Hardman, T.C.; Dubrey, S.W. Development and potential role of type-2 sodium-glucose transporter inhibitors for management of type 2 diabetes. Diabetes Ther. 2011, 2, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Devineni, D.; Morrow, L.; Hompesch, M.; Skee, D.; Vandebosch, A.; Murphy, J.; Ways, K.; Schwartz, S. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes. Metab. 2012, 14, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Sakamaki, S.; Hongu, M.; Kawanishi, E.; Koga, Y.; Sakamoto, T.; Yamamoto, Y.; Ueta, K.; Kimata, H.; Nakayama, K.; et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J. Med. Chem. 2010, 53, 6355–6360. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Devineni, D.; Ghosh, A.; Polidori, D.; Chien, S.; Wexler, D.; Shalayda, K.; Demarest, K.; Rothenberg, P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 2011, 13, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Arakawa, K.; Ueta, K.; Matsushita, Y.; Kuriyama, C.; Martin, T.; Du, F.; Liu, Y.; Xu, J.; Conway, B.; et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS ONE 2012, 7, e30555. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Aggarwal, N.; Polidori, D.; Zhao, Y.; Arbit, D.; Usiskin, K.; Capuano, G.; Canovatchel, W. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012, 35, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Forst, T.; Guthrie, R.; Goldenberg, R.; Yee, J.; Vijapurkar, U.; Meininger, G.; Stein, P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes. Metab. 2014, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Polidori, D.; Sha, S.; Mudaliar, S.; Ciaraldi, T.P.; Ghosh, A.; Vaccaro, N.; Farrell, K.; Rothenberg, P.; Henry, R.R. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: Results of a randomized, placebo-controlled study. Diabetes Care 2013, 36, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, R.N.; Cuyckens, F.; Chen, J.; Scheers, E.; Kalamaridis, D.; Lin, R.; Silva, J.; Sha, S.; Evans, D.C.; Kelley, M.F.; et al. Metabolism and excretion of canagliflozin in mice, rats, dogs, and humans. Drug Metab. Dispos. 2014, 42, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Devineni, D.; Vaccaro, N.; Murphy, J.; Curtin, C.; Mamidi, R.N.; Weiner, S.; Wang, S.S.; Ariyawansa, J.; Stieltjes, H.; Wajs, E.; et al. Effects of rifampin, cyclosporine A, and probenecid on the pharmacokinetic profile of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Int. J. Clin. Pharmacol. Ther. 2015, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Devineni, D.; Polidori, D. Clinical Pharmacokinetic, Pharmacodynamic, and Drug-Drug Interaction Profile of Canagliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor. Clin. Pharmacogenet. 2015, 54, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Steen, O.; Goldenberg, R.M. The Role of Sodium-Glucose Cotransporter 2 Inhibitors in the Management of Type 2 Diabetes. Can. J. Diabetes 2017, 41, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ezzeldin, E.; Al-Rashood, K.A.; Asiri, Y.A.; Rezk, N.L. Rapid determination of canagliflozin in rat plasma by UHPLC-MS/MS using negative ionization mode to avoid adduct-ions formation. Talanta 2015, 132, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, S.; Yano, K.; Ito, Y.; Sakaeda, T. A validated LC-MS/MS method for the determination of canagliflozin, a sodium-glucose co-transporter 2 (SGLT-2) inhibitor, in a lower volume of rat plasma: Application to pharmacokinetic studies in rats. Biomed. Chromatogr. 2016, 30, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Liang, Y.; Jiye, A.; Hao, H.; Liu, L.; Zheng, X.; Dai, C.; Zhou, Y.; Guan, T.; Liu, Y.; et al. Post acquisition data processing techniques for lipid analysis by quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2012, 905, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, L.; Leng, J.; Chen, P.; Fan, X.; Cheng, Y. Fragment ion diagnostic strategies for the comprehensive identification of chemical profile of Gui-Zhi-Tang by integrating high-resolution MS, multiple-stage MS and UV information. J. Pharm. Biomed. Anal. 2014, 98, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Qiao, X.; Wang, Q.; Li, R.; Miao, W.; Guo, D.; Ye, M. From single compounds to herbal extract: A strategy to systematically characterize the metabolites of licorice in rats. Drug Metab. Dispos. 2011, 39, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Kondo, K.; Yoshinari, T.; Ishii, M.; Sakai, M.; Kuki, H.; Furihata, K. Pharmacokinetic and pharmacodynamic profiles of canagliflozin in Japanese patients with type 2 diabetes mellitus and moderate renal impairment. Clin. Drug Investig. 2014, 34, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Kifuji, T.; Maruyama, N.; Inagaki, N. Pharmacokinetics, Pharmacodynamics, and Safety of Canagliflozin in Japanese Patients with Type 2 Diabetes Mellitus. Adv. Ther. 2015, 32, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Lavalle-Gonzalez, F.J.; Januszewicz, A.; Davidson, J.; Tong, C.; Qiu, R.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia 2013, 56, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Kabil, S.L.; Mahmoud, N.M. Canagliflozin protects against non-alcoholic steatohepatitis in type-2 diabetic rats through zinc alpha-2 glycoprotein up-regulation. Eur. J. Pharmacol. 2018, 828, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Emi, Y.; Ikushiro, S.; Iyanagi, T. Drug-responsive and tissue-specific alternative expression of multiple first exons in rat UDP-glucuronosyltransferase family 1 (UGT1) gene complex. J. Biochem. 1995, 117, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.J.; Miles, K.K.; Auyeung, D.J.; Kessler, F.K.; Ritter, J.K. Analysis of substrate specificities and tissue expression of rat UDP-glucuronosyltransferases UGT1A7 and UGT1A8. Drug Metab. Dispos. 2005, 33, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wu, J.; Meng, C.; Ma, B.; Wang, T.; Li, Y.; Ma, Y. Cyp3a11-mediated testosterone-6beta-hydroxylation decreased, while UGT1a9-mediated propofol O-glucuronidation increased, in mice with diabetes mellitus. Biopharm. Drug Dispos. 2016, 37, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Sun, S.; Cheng, X.; Yan, T.; Zheng, X.; Li, F.; Qi, Q.; Wang, G.; Hao, H. Dysregulations of intestinal and colonic UDP-glucuronosyltransferases in rats with type 2 diabetes. Drug Metab. Biopharm. 2013, 28, 427–434. [Google Scholar] [CrossRef]
- Hu, N.; Hu, M.; Duan, R.; Liu, C.; Guo, H.; Zhang, M.; Yu, Y.; Wang, X.; Liu, L.; Liu, X. Increased levels of fatty acids contributed to induction of hepatic CYP3A4 activity induced by diabetes—In vitro evidence from HepG2 cell and Fa2N-4 cell lines. J. Pharmacol. Sci. 2014, 124, 433–444. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds puerarin are available from the authors. |









| Analyte | Concentration (ng/L) | Extraction Recovery (%) | Intra-Day | Inter-Day | Accuracy (%) | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/L) | RSD (%) | Mean ± SD (ng/L) | RSD (%) | ||||
| Canagliflozin | 800 | 92.11 ± 5.31 | 786.44 ± 15.33 | 1.95 | 802.54 ± 13.22 | 1.65 | 6.12 |
| 500 | 93.18 ± 3.11 | 503.19 ± 19.17 | 3.81 | 502.97 ± 17.79 | 3.53 | 4.08 | |
| 25 | 91.17 ± 2.14 | 26.44 ± 2.55 | 9.64 | 26.63 ± 1.95 | 7.32 | 3.26 | |
| 10 | 92.05 ± 1.51 | 11.56 ± 1.09 | 9.43 | 11.42 ± 1.22 | 10.68 | 4.13 | |
| Storage Conditions | Concentration (ng/L) | Mean ± SD | RSD % |
|---|---|---|---|
| Autosampler (4 °C) temperature for 24 h | 25 | 25.72 ± 0.33 | 1.28 |
| 500 | 503.15 ± 4.41 | 0.87 | |
| 800 | 806.81 ± 5.64 | 0.69 | |
| Room temperature (25 °C) for 24 h | 25 | 26.21 ± 0.96 | 3.66 |
| 500 | 504.45 ± 3.18 | 0.63 | |
| 800 | 807.28 ± 6.12 | 0.76 | |
| −80 °C temperature for 30 days | 25 | 26.89 ± 0.85 | 3.16 |
| 500 | 504.33 ± 2.14 | 0.42 | |
| 800 | 806.34 ± 3.51 | 0.44 | |
| Three freeze–thaw cycles (each at −80 °C for 24 h) | 25 | 25.97 ± 1.02 | 3.93 |
| 500 | 505.20 ± 4.12 | 0.82 | |
| 800 | 806.17 ± 4.19 | 0.52 |
| Parameters | CTRL | DM |
|---|---|---|
| Serum triglyceride (mmol/L) | 2.53 ± 0.32 | 4.97 ± 0.61 * |
| Serum total cholesterol (mmol/L) | 2.21 ± 0.65 | 5.12 ± 1.07 * |
| Initial serum glucose (mmol/L) | 6.96 ± 0.69 | 7.12 ± 0.85 |
| Final serum glucose (mmol/L) | 7.01 ± 0.87 | 32.43 ± 3.23 ** |
| HOMA-IR | 6.02 ± 2.13 | 21.76 ± 11.14 ** |
| Initial body weight (g) | 221.46 ± 13.08 | 239.51 ± 15.02 |
| Final body weight (g) | 375.51 ± 29.67 | 387.31 ± 32.87 |
| Parameters | Pharmacokinetic Parameters | CTRL | DM |
|---|---|---|---|
| Canagliflozin | AUC0–t (ng·h/L) | 51,988.40 ± 4162.72 | 27,116.66 ± 2694.322 ** |
| AUC0–∞ (ng·h/L) | 56,314.67 ± 5095.46 | 28,423.95 ± 3072.73 ** | |
| MRT0–t (h) | 14.08 ± 1.58 | 12.35 ± 2.77 | |
| t1/2z (h) | 12.86 ± 2.64 | 10.24 ± 3.49 * | |
| Tmax (h) | 3.6 ± 0.89 | 3.20 ± 0.45 | |
| CLz/F (L/h/kg) | 357.46 ± 32.06 | 710.37 ± 77.98 ** | |
| Cmax (ng/L) | 3310.00 ± 566.13 | 2226.00 ± 559.31 ** | |
| Vz/F (L/kg) | 6600.39 ± 1372.71 | 10,508.36 ± 3544.44 ** | |
| M5 | AUC0–t (ng·h/L) | 917.27 ± 227.42 | 1461.12 ± 371.70 ** |
| t1/2z (h) | 14.63 ± 18.29 | 13.32 ± 6.25 | |
| Tmax (h) | 1.80 ± 0.84 | 1.67 ± 0.58 | |
| Cmax (ng/L) | 126.42 ± 31.05 | 160.12 ± 54.34 * | |
| M7 | AUC0–t (ng·h/L) | 215.79 ± 26.95 | 501.35 ± 143.93 ** |
| t1/2z (h) | 10.04 ± 6.45 | 14.48 ± 4.86 | |
| Tmax (h) | 2.02 ± 0.11 | 1.81 ± 0.12 | |
| Cmax (ng/L) | 44.89 ± 20.5 | 90.53 ± 18.65 ** | |
| M17 | AUC0–t (ng·h/L) | 58.80 ± 17.92 | 91.81 ± 24.22 ** |
| t1/2z (h) | 9.95 ± 3.57 | 9.02 ± 6.75 | |
| Tmax (h) | 2.6 ± 0.89 | 2.33 ± 0.58 | |
| Cmax (ng/L) | 7.27 ± 2.36 | 11.74 ± 2.37 * | |
| M9 | AUC0–t (ng·h/L) | 929.35 ± 226.29 | 1631.37 ± 131.63 ** |
| t1/2z (h) | 10.74 ± 1.63 | 12.78 ± 8.81 | |
| Tmax (h) | 5.01 ± 0.11 | 4.75 ± 0.5 | |
| Cmax (ng/L) | 97.49 ± 10.84 | 160.5 ± 10.48 * |
| Analyte | Q1 (m/z) | Q3 (m/z) | Declustering Potential (V) | Entrance Potential (V) | Collision Energy (V) | Collision Cell Exit Potential (V) |
|---|---|---|---|---|---|---|
| Canagliflozin | 462.00 | 191.10 | 40.00 | 6.50 | 41.00 | 10.00 |
| Empagliflozin (IS) | 451.20 | 71.10 | 130.00 | 6.50 | 31.00 | 10.00 |
| M5 | 638.00 | 191.10 | 50.00 | 6.50 | 30.00 | 10.00 |
| M7 | 638.00 | 191.10 | 50.00 | 6.50 | 30.00 | 10.00 |
| M17 | 638.00 | 191.10 | 50.00 | 6.50 | 30.00 | 10.00 |
| M9 | 478.00 | 267.00 | 50.00 | 6.50 | 30.00 | 10.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.-t.; Niu, H.-m.; Wu, Y.; Jiang, J.-l.; Li, Y.; Jiang, K.-y.; Wang, X.; Zhang, M.-f.; Han, M.-f.; Meng, S.-n. Plasma Pharmacokinetic Determination of Canagliflozin and Its Metabolites in a Type 2 Diabetic Rat Model by UPLC-MS/MS. Molecules 2018, 23, 1229. https://doi.org/10.3390/molecules23051229
Dong S-t, Niu H-m, Wu Y, Jiang J-l, Li Y, Jiang K-y, Wang X, Zhang M-f, Han M-f, Meng S-n. Plasma Pharmacokinetic Determination of Canagliflozin and Its Metabolites in a Type 2 Diabetic Rat Model by UPLC-MS/MS. Molecules. 2018; 23(5):1229. https://doi.org/10.3390/molecules23051229
Chicago/Turabian StyleDong, Song-tao, Hui-min Niu, Yin Wu, Jia-lei Jiang, Ying Li, Kun-yu Jiang, Xin Wang, Mao-fan Zhang, Ming-feng Han, and Sheng-nan Meng. 2018. "Plasma Pharmacokinetic Determination of Canagliflozin and Its Metabolites in a Type 2 Diabetic Rat Model by UPLC-MS/MS" Molecules 23, no. 5: 1229. https://doi.org/10.3390/molecules23051229