Cellulose Nanofiber Biotemplated Palladium Composite Aerogels
Abstract
:1. Introduction
2. Materials and Methods
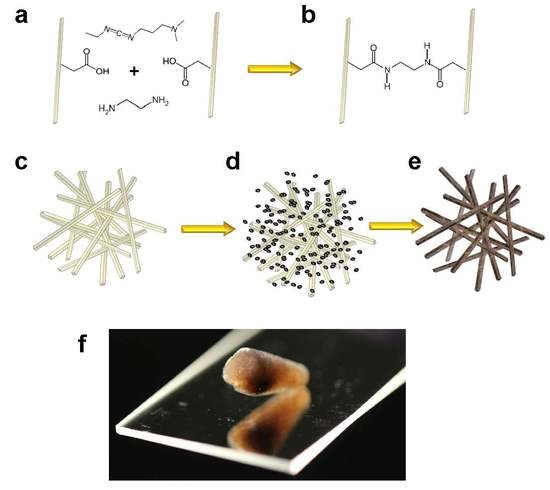
2.1. Hydrogel Preparation
2.2. Scanning Electron Microscopy
2.3. Thermal Gravimetric Analysis (TGA)
2.4. X-Ray Diffractometry
2.5. BET Analysis
2.6. Electrochemical Characterization
3. Results and Discussion
3.1. Aerogel Synthesis
3.2. Scanning Electron Microscopy
3.3. X-Ray Diffractometry
3.4. Thermalgravimetric Analysis (TGA)
3.5. Nitrogen Gas Adsorption
3.6. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sotiropoulou, S.; Sierra-Sastre, Y.; Mark, S.S.; Batt, C.A. Biotemplated Nanostructured Materials. Chem. Mater. 2008, 20, 821–834. [Google Scholar] [CrossRef]
- Bashir, R. BioMEMS: State-of-the-Art in Detection, Opportunities and Prospects. Advanc. Drug Deliv. Rev. 2004, 56, 1565–1586. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Marelli, B.; Brenckle, M.A.; Mitropoulos, A.N.; Gil, E.-S.; Tsioris, K.; Tao, H.; Kaplan, D.L.; Omenetto, F.G. All-Water-Based Electron-Beam Lithography Using Silk as a Resist. Nat. Nanotechnol. 2014, 9, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Timms, P.L.; Göltner-Spickermann, C. Exact Replication of Biological Structures by Chemical Vapor Deposition of Silica. Angew. Chemie Int. Ed. 2003, 42, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kim, H.J.; Xu, P.; Matsumoto, A.; Kaplan, D.L. Biomaterial Coatings by Stepwise Deposition of Silk Fibroin. Langmuir 2005, 21, 11335–11341. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kundu, D.; Uyama, H. One-Pot Fabrication of Palladium Nanoparticles Captured in Mesoporous Polymeric Monoliths and Their Catalytic Application in C-C Coupling Reactions. J. Colloid Interface Sci. 2015, 451, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Cirtiu, C.M.; Dunlop-Brière, A.F.; Moores, A. Cellulose Nanocrystallites as an Efficient Support for Nanoparticles of Palladium: Application for Catalytichydrogenation and Heck Coupling under Mild Conditions. Green Chem. 2011, 13, 288–291. [Google Scholar] [CrossRef]
- Jarvis, M. Cellulose Stacks up. Nature. 2003, 426, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional Nanofibers for Environmental Applications. J. Mater. Chem. 2008, 18, 5326. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated Cellulose and New Nanocomposite Materials: A Review. Cellulose. 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Takagi, H.; Asano, A. Effects of Processing Conditions on Flexural Properties of Cellulose Nanofiber Reinforced “green” composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 685–689. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent Advances in the Application of Cellulose Nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Ramires, E.C.; Dufresne, A. A Review of Cellulose Nanocrystals and Nanocomposites. Tappi J. 2011, 10, 9–16. [Google Scholar]
- Wang, J.; Zhang, J.; Han, J. Synthesis of Crystals and Particles by Crystallization and Polymerization in Droplet-Based Microfluidic Devices. Front. Chem. Eng. China 2010, 4, 26–36. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in Biomedicine: Current Status and Future Prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent Advances in Nanocellulose for Biomedical Applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose Using Various Mechanical Processes: A Review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials (Basel). 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing Bulk Natural Wood into a High-Performance Structural Material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as Sustainable Biomass Material: Structure, Properties, Present Status and Future Prospects in Biomedical Applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shim, B.S.; Kim, H.S.; Lee, Y.J.; Min, S.K.; Jang, D.; Abas, Z.; Kim, J. Review of Nanocellulose for Sustainable Future Materials. Int. J. Precis. Eng. Manuf.-Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Fischer, F.; Rigacci, A.; Pirard, R.; Berthon-Fabry, S.; Achard, P. Cellulose-Based Aerogels. Polym. (Guildf). 2006, 47, 7636–7645. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar Cellulose Aerogels. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-Porosity Aerogels of High Specific Surface Area Prepared from Nanofibrillated Cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Yano, H. Surface Modification of Bacterial Cellulose Nanofibers for Property Enhancement of Optically Transparent Composites: Dependence on Acetyl-Group DS. Biomacromolecules 2007, 8, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, T.; He, Z.; Zhong, A.; Huang, K. Carboxyl-Containing Microporous Organic Nanotube Networks as a Platform for Pd Catalysts. RSC Adv. 2016, 6, 39933–39939. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.; Fu, S.; Chen, J.; Berry, R.M.; Tam, K.C. Strategy for Synthesizing Porous Cellulose Nanocrystal Supported Metal Nanocatalysts. ACS Sustain. Chem. Eng. 2016, 4, 5929–5935. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Xu, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B.; Sui, X. Cellulose Sponge Supported Palladium Nanoparticles as Recyclable Cross-Coupling Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 17155–17162. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous Cellulose as Metal Nanoparticles Support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.H.; Hinestroza, J.P. Metal Nanoparticles on Natural Cellulose Fibers: Electrostatic Assembly and in Situ Synthesis. ACS Appl. Mater. Interfaces 2009, 1, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Song, L.T.; Wu, Z.Y.; Liang, H.W.; Zhou, F.; Yu, Z.Y.; Xu, L.; Pan, Z.; Yu, S.H. Macroscopic-Scale Synthesis of Nitrogen-Doped Carbon Nanofiber Aerogels by Template-Directed Hydrothermal Carbonization of Nitrogen-Containing Carbohydrates. Nano Energy 2016, 19, 117–127. [Google Scholar] [CrossRef]
- Ingale, S.V.; Sastry, P.U.; Wagh, P.B.; Tripathi, A.K.; Rao, R.; Tewari, R.; Rao, P.T.; Patel, R.P.; Tyagi, A.K.; Gupta, S.C. Synthesis and Micro Structural Investigations of Titania-Silica Nano Composite Aerogels. Mater. Chem. Phys. 2012, 135, 497–502. [Google Scholar] [CrossRef]
- Zielasek, V.; Jürgens, B.; Schulz, C.; Biener, J.; Biener, M.M.; Hamza, A.V.; Bäumer, M. Gold Catalysts: Nanoporous Gold Foams. Angew. Chemie-Int. Ed. 2006, 45, 8241–8244. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Jung, H.Y.; Fang, W.; Dresselhaus, M.S.; Kong, J. A Facile Methodology for the Production of in Situ Inorganic Nanowire Hydrogels/aerogels. Nano Lett. 2014, 14, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; McClure, J.P.; Ryu, M.Y.; Palmer, J.L. Direct Solution-Based Reduction Synthesis of Au, Pd, and Pt Aerogels. J. Mater. Res. 2017, 32, 4153–4165. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Yuan, G.; Yin, C.; Song, K.H.; Lee, J.S.; Chung, I.M.; Karvembu, R.; Kim, I.S. Noble Metal/functionalized Cellulose Nanofiber Composites for Catalytic Applications. Carbohydr. Polym. 2015, 132, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Park, J.Y.; Renzas, J.R.; Butcher, D.R.; Huang, W.; Somorjai, G.A. Size Effect of Ruthenium Nanoparticles in Catalytic Carbon Monoxide Oxidation. Nano Lett. 2010, 10, 2709–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.M.; Yu, W.Y.; Che, C.M. Ruthenium Nanoparticles Supported on Hydroxyapatite as an Efficient and Recyclable Catalyst for Cis-Dihydroxylation and Oxidative Cleavage of Alkenes. Angew. Chemie-Int. Ed. 2004, 43, 3303–3307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyczyk, A.; Sniechota, A.; Adamczyk, A.; Bernasik, A.; Turek, W.; Hasik, M. Investigations of Polyaniline-Platinum Composites Prepared by Sodium Borohydride Reduction. Eur. Polym. J. 2008, 44, 1594–1602. [Google Scholar] [CrossRef]
- Williams, J.R.; Clifford, A.A.; al-Saidi, S.H.R. Supercritical Fluids and Their Applications in Biotechnology and Related Areas. Mol. Biotechnol. 2002, 22, 263–286. [Google Scholar] [CrossRef]
- Cooper, A. Clean Polymer Synthesis and Processing Using Supercritical Carbon Dioxide. Green Chem. 1999, 1, G167–G168. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Craver, C.D.; Charles, E.; Carraher, J.; Boffa, L.S. Applied Polymer Science: 21st Century; Elsevier Science: Amsterdam, Netherlands, 2000; pp. 305–316. [Google Scholar]
- Sannino, A.; Pappadà, S.; Madaghiele, M.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Crosslinking of Cellulose Derivatives and Hyaluronic Acid with Water-Soluble Carbodiimide. Polym. (Guildf). 2005, 46, 11206–11212. [Google Scholar] [CrossRef]
- Jewell, L.L.; Davis, B.H. Review of Absorption and Adsorption in the Hydrogen-Palladium System. Appl. Catalysis A: Gen. 2006, 310, 1–15. [Google Scholar] [CrossRef]
- Borsoi, C.; Zimmernnam, M.V.G.; Zattera, A.J.; Santana, R.M.C.; Ferreira, C.A. Thermal Degradation Behavior of Cellulose Nanofibers and Nanowhiskers. J. Therm. Anal. Calorim. 2016, 126, 1867–1878. [Google Scholar] [CrossRef]
- Bisquert, J. Infuence of the boundaries in the impedance of porous film electrodes. Phys. Chem. Chem. Phys. 2000, 2, 4185–4192. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the Impedance of Electron Diffusion and Recombination in a Thin Layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhoes, L.O.S. Impedance of constant phase element (CPE)-blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar] [CrossRef]
Sample Availability: Samples of the CNF-Pd aerogels are available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burpo, F.J.; Mitropoulos, A.N.; Nagelli, E.A.; Palmer, J.L.; Morris, L.A.; Ryu, M.Y.; Wickiser, J.K. Cellulose Nanofiber Biotemplated Palladium Composite Aerogels. Molecules 2018, 23, 1405. https://doi.org/10.3390/molecules23061405
Burpo FJ, Mitropoulos AN, Nagelli EA, Palmer JL, Morris LA, Ryu MY, Wickiser JK. Cellulose Nanofiber Biotemplated Palladium Composite Aerogels. Molecules. 2018; 23(6):1405. https://doi.org/10.3390/molecules23061405
Chicago/Turabian StyleBurpo, Fred J., Alexander N. Mitropoulos, Enoch A. Nagelli, Jesse L. Palmer, Lauren A. Morris, Madeline Y. Ryu, and J. Kenneth Wickiser. 2018. "Cellulose Nanofiber Biotemplated Palladium Composite Aerogels" Molecules 23, no. 6: 1405. https://doi.org/10.3390/molecules23061405