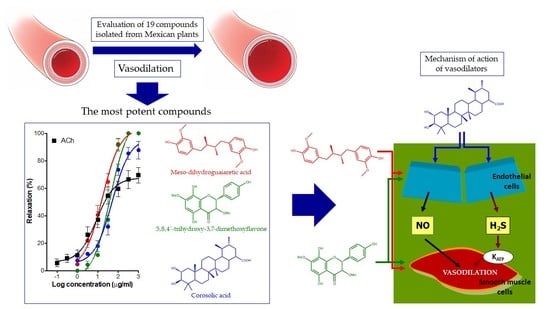
Vasodilator Activity of Compounds Isolated from Plants Used in Mexican Traditional Medicine
Abstract
:1. Introduction
2. Results
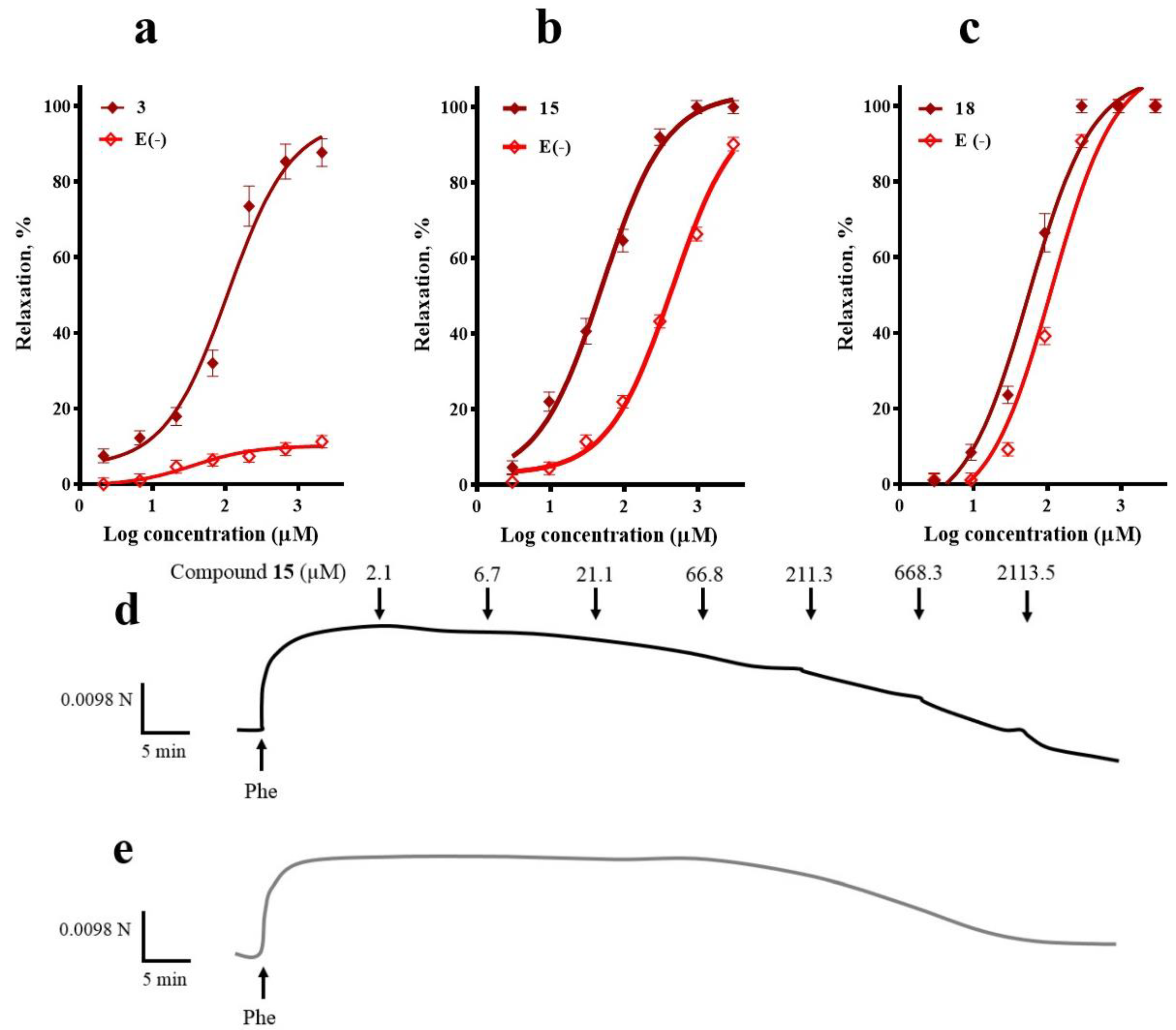
2.1. Participation of the Endothelium in the Vasorelaxant Response of Compounds 3, 15, and 18
2.2. Participation of the NO/cGMP and H2S/KATP Channel Pathways in the Vasodilator Response of Compounds 3, 15, and 18
2.3. Involvement of K+ Channels in the Vasodilation Evoked by Compounds 3, 15, and 18
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Isolation, Purification and Structural Characterization of Phytochemicals
4.3. Experimental Animals
4.4. Determination of the Vasodilator Effect of the Selected Secondary Metabolites
4.4.1. Isolated Rat Aorta Assay
4.4.2. Participation of the Endothelium in the Vasorelaxant Response of Compounds 3, 15, and 18
4.5. Evaluation of the Participation of the NO/cGMP and H2S/KATP Channel Pathways in the Vasodilator Response of Compounds 3, 15, and 18
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef]
- Wu, F.; Guo, Y.; Chatterji, S.; Zheng, Y.; Naidoo, N.; Jiang, Y.; Biritwum, R.; Yawson, A.; Minicuci, N.; Salinas-Rodriguez, A. Common risk factors for chronic non-communicable diseases among older adults in China, Ghana, Mexico, India, Russia and South Africa: The study on global AGEing and adult health (SAGE) wave 1. BMC Public Health 2015, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Top Ten Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 17 February 2017).
- Bakris, G.; Sarafidis, P.; Agarwal, R.; Ruilope, L. Review of blood pressure control rates and outcomes. J. Am. Soc. Hypertens. 2014, 8, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global disparities of hypertension prevalence and control. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Düsing, R.; Waeber, B.; Destro, M.; Maia, C.S.; Brunel, P. Triple-combination therapy in the treatment of hypertension: A review of the evidence. J. Hum. Hypertens. 2017, 31, 501. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-T. Endothelium-derived relaxing factors of small resistance arteries in hypertension. Toxicol. Res. 2014, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Simopoulou, C.; Papageorgiou, N.; Oikonomou, E.; Hatzis, G.; Siasos, G.; Tsiamis, E.; Stefanadis, C. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol. Ther. 2014, 144, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Patel, R.P.; Kevil, C.G. Working with nitric oxide and hydrogen sulfide in biological systems. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L403–L415. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.; Shimokawa, H.; Feletou, M.; Tang, E. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F. Historia Natural de Nueva España; Universidad Nacional de México: Mexico D.F., Mexico, 1959. (In Spanish) [Google Scholar]
- De la Cruz, M. Libellus de medicinalibus indorum herbis: Aztec manuscript of 1552 (as latin traduction of Juan Badiano); Fondo de Cultura Economica; Instituto Mexicano del Seguro Social: Mexico D.F., Mexico, 1991. (In Spanish) [Google Scholar]
- Díaz, J. Uso de las Plantas Medicinales de México; Instituto Mexicano para el Estudio de las Plantas Medicinales: Mexico D.F., Mexico, 1976; p. 329. (In Spanish) [Google Scholar]
- Camacho, M.d.R.; Mata, R.; Castaneda, P.; Kirby, G.C.; Warhurst, D.C.; Croft, S.L.; Phillipson, J.D. Bioactive compounds from Celaenodendron mexicanum. Planta Med. 2000, 66, 463–468. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Delgado, G. Uncommon sesquiterpenoids and new triterpenoids from Jatropha neopauciflora (Euphorbiaceae). Helvetica Chimica Acta 2006, 89, 16–29. [Google Scholar] [CrossRef]
- Biblioteca Digital de la Medicina Tradicional Mexicana. Available online: http://www.medicinatradicionalmexicana.unam.mx/index.php (accessed on 30 January 2018).
- Hernández-Hernández, A.; Alarcón-Aguilar, F.; Jiménez-Estrada, M.; Hernández-Portilla, L.; Flores-Ortiz, C.; Rodríguez-Monroy, M.; Canales-Martínez, M. Biological properties and chemical composition of Jatropha neopauciflora pax. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, J.; Siles-Barrios, D.; García-Sánchez, J.; Reyes-Trejo, B.; Sánchez-Mendoza, M.E. Relaxant effect of the extracts of Crataegus mexicana on guinea pig tracheal smooth muscle. Pharmacogn. J. 2010, 2, 40–46. [Google Scholar] [CrossRef]
- Estrada-Muñiz, E.; Guerrero-Palomo, G.; Vega, L. Natural products: New anti-cancer agents derived from plants. Curr. Top. Toxicol. 2012, 8, 19–32. [Google Scholar]
- Camacho, M.D.R.; Phillipson, J.D.; Croft, S.L.; Marley, D.; Kirby, G.C.; Warhurst, D.C. Assessment of the antiprotozoal activity of Galphimia glauca and the isolation of new nor-secofriedelanes and nor-friedelanes. J. Nat. Prod. 2002, 65, 1457–1461. [Google Scholar] [CrossRef]
- Favela-Hernández, J.; García, A.; Garza-González, E.; Rivas-Galindo, V.; Camacho-Corona, M.d.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, A.; Bah, M.; Ibarra-Alvarado, C.; Rivero-Cruz, J.F.; Rojas-Molina, A.; Rojas-Molina, J.I.; Cabrera-Luna, J.A. Aortic relaxant activity of Crataegus gracilior Phipps and identification of some of its chemical constituents. Molecules 2014, 19, 20962–20974. [Google Scholar] [CrossRef] [PubMed]
- Canales, M.; Hernández, T.; Caballero, J.; De Vivar, A.R.; Avila, G.; Duran, A.; Lira, R. Informant consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlán, Puebla, México. J. Ethnopharmacol. 2005, 97, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.D.R.; Sanchez, B.; Quiroz, H.; Contreras, J.L.; Mata, R. Pinocembrine: A bioactive flavanone from Teloxys graveolens. J. Ethnopharmacol. 1991, 31, 383–389. [Google Scholar] [CrossRef]
- Calzada, F.; Velázquez, C.; Cedillo-Rivera, R.; Esquivel, B. Antiprotozoal activity of the constituents of Teloxys graveolens. Phytother. Res. 2003, 17, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Cha, J.-Y.; Kang, H.S.; Lee, J.-H.; Lee, J.Y.; Park, J.-H.; Bae, J.-H.; Song, D.-K.; Im, S.-S. Corosolic acid ameliorates acute inflammation through inhibition of IRAK-1 phosphorylation in macrophages. BMB Rep. 2016, 49, 276. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Tian, W.; Liu, X.-X.; Zhang, K.; Huo, J.-C.; Liu, W.-J.; Li, P.; Xiao, X.; Zhao, M.-G.; Cao, W. Corosolic acid inhibits the proliferation of glomerular mesangial cells and protects against diabetic renal damage. Sci. Rep. 2016, 6, 26854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M.Á. Vasodilator compounds derived from plants and their mechanisms of action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Romo-Mancillas, A.; López-Vallejo, F.H.; Solís-Gutiérrez, M.; Rojas-Molina, J.I.; Rivero-Cruz, F. Role of nitric oxide and hydrogen sulfide in the vasodilator effect of ursolic acid and uvaol from black cherry Prunus serotina fruits. Molecules 2016, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Navarrete-Vazquez, G.; Hidalgo-Figueroa, S.; Hernández-Abreu, O.; Estrada-Soto, S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: Ex vivo and in silico studies. Fitoterapia 2012, 83, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.W.; Choi, C.Y.; Hwang, Y.P.; Kim, H.G.; Kim, S.J.; Chung, Y.C.; Lee, K.J.; Jeong, T.C.; Jeong, H.G. Betulinic acid increases eNOS phosphorylation and no synthesis via the calcium-signaling pathway. J. Agric. Food Chem. 2016, 64, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Madlala, H.P.; Metzinger, T.; van Heerden, F.R.; Musabayane, C.T.; Mubagwa, K.; Dessy, C. Vascular endothelium-dependent and independent actions of oleanolic acid and its synthetic oleanane derivatives as possible mechanisms for hypotensive effects. PLoS ONE 2016, 11, e0147395. [Google Scholar] [CrossRef] [PubMed]
- White, R.E.; Kryman, J.P.; El-Mowafy, A.M.; Han, G.; Carrier, G.O. Camp-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BKCa channel activity in coronary artery smooth muscle cells. Circ. Res. 2000, 86, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Boerth, N.J.; Dey, N.B.; Cornwell, T.L.; Lincoln, T.M. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J. Vasc. Res. 1997, 34, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, H.; Liu, P.; Tang, C.; Wang, J. Hydrogen sulfide contributes to cardioprotection during ischemia–reperfusion injury by opening KATP channels. Can. J. Physiol. Pharmacol. 2007, 85, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Jupiter, R.C.; Pankey, E.A.; Reddy, V.G.; Edward, J.A.; Swan, K.W.; Peak, T.C.; Mostany, R.; Kadowitz, P.J. Analysis of cardiovascular responses to the H2S donors na Na2S and NaHS in the rat. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H605–H614. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, K.; Seiringer, G.n.; Nguyen, D.L.; Winkler, J.; Blaschke, M.; McKinnon, R.; Urban, E.; Ladurner, A.; Dirsch, V.M.; Zehl, M. Triterpenoic acids from apple pomace enhance the activity of the endothelial nitric oxide synthase (eNOS). J. Agric. Food Chem. 2015, 64, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hosokawa, M.; Yamada, C.; Watanabe, R.; Fujimoto, S.; Fujiwara, H.; Kunitomo, M.; Miura, T.; Kaneko, T.; Tsuda, K. Dietary corosolic acid ameliorates obesity and hepatic steatosis in KK-Ay mice. Biol. Pharm. Bull. 2008, 31, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, Y.H.; Song, G.-Y.; Kim, D.-E.; Jeong, Y.-J.; Liu, K.-H.; Chung, Y.-H.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food Chem. Toxicol. 2014, 67, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Yamada, K.; Yoshikawa, N.; Nakamura, K.; Haginaka, J.; Kunitomo, M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006, 79, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol.-Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Martin, E.; Sharina, I.; Esposito, I.; Szabo, C.; Bucci, M.; Cirino, G.; Papapetropoulos, A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol. Res. 2016, 111, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bełtowski, J.; Jamroz-Wiśniewska, A. Hydrogen sulfide and endothelium-dependent vasorelaxation. Molecules 2014, 19, 21183–21199. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.L.; Lumsden, N.G.; Farry, J.; Jefferis, A.-M.; Kemp-Harper, B.K.; Chin-Dusting, J.P. Nitroxyl: A vasodilator of human vessels that is not susceptible to tolerance. Clin. Sci. 2015, 129, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Nagpure, B.; Bian, J.-S. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid. Med. Cell. Longev. 2016, 6904327. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.J.; Kim, S.R.; Kim, J.; Kim, Y.C. Meso-dihydroguaiaretic acid and licarin a of Machilus thunbergii protect against glutamate-induced toxicity in primary cultures of a rat cortical cells. Br. J. Pharmacol. 2005, 146, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Seo, C.S.; Haa, K.; Kim, J.C.; Hwang, N.K.; Hong, T.G.; Kim, J.H.; Kim, D.H.; Son, J.K.; Chang, H.W. Meso-dihydroguaiaretic acid isolated from Saururus chinensis inhibits cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2008, 31, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Trang, T.T.T. Compounds from the aerial part of Saururus chinensis and their cytotoxic activity. Nat. Prod. Sci. 2012, 18, 227–232. [Google Scholar]
- Choi, M.S.; Jeong, H.J.; Kang, T.-h.; Shin, H.-M.; Oh, S.T.; Choi, Y.; Jeon, S. Meso-dihydroguaiaretic acid induces apoptosis and inhibits cell migration via p38 activation and EGFR/Src/intergrin β3 downregulation in breast cancer cells. Life Sci. 2015, 141, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E. Cardiovascular activity of naturally occurring lignans. Phytomedicine 1997, 4, 151–166. [Google Scholar] [CrossRef]
- Song, M.-C.; Kim, E.-C.; Kim, W.-J.; Kim, T.-J. Meso-dihydroguaiaretic acid inhibits rat aortic vascular smooth muscle cell proliferation by suppressing phosphorylation of platelet-derived growth factor receptor beta. Eur. J. Pharmacol. 2014, 744, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Drummond, G.R.; Ahn, J.; Storek, M.; Pohl, J.; Parthasarathy, S.; Harrison, D.G. Modulation of expression of endothelial nitric oxide synthase by nordihydroguaiaretic acid, a phenolic antioxidant in cultured endothelial cells. Mol. Pharmacol. 1999, 56, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Nagano, N.; Hirano, M.; Muraki, K.; Watanabe, M.; Imaizumi, Y. Activation of Ca2+-dependent K+ current by nordihydroguaiaretic acid in porcine coronary arterial smooth muscle cells. J. Pharmacol. Exp. Ther. 1999, 291, 140–146. [Google Scholar] [PubMed]
- Yamamura, H.; Sakamoto, K.; Ohya, S.; Muraki, K.; Imaizumi, Y. Mechanisms underlying the activation of large conductance Ca2+-activated K+ channels by nordihydroguaiaretic acid. Jap. J. Pharmacol. 2002, 89, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 370, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morán-Pinzón, J.; Mondolis, E.; Abad, A.; Amaro-Luis, J.M.; Sevilla, M.Á.; Montero, M.J.; López-Pérez, J.L.; Guerrero De León, E. Vasorelaxan effect and potent antioxidant activity of natural flavones isolated from Lourteigia stoechadifolia and Ageratina stevioides, two venezuelan plants. Eur. J. Med. Plants 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Bertin, R.; Chen, Z.; Martinez-Vazquez, M.; Garcia-Argaez, A.; Froldi, G. Vasodilation and radical-scavenging activity of imperatorin and selected coumarinic and flavonoid compounds from genus Casimiroa. Phytomedicine 2014, 21, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Tep-areenan, P.; Sawasdee, P.; Randall, M. Possible mechanisms of vasorelaxation for 5, 7-dimethoxyflavone from Kaempferia parviflora in the rat aorta. Phytother. Res. 2010, 24, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Tan, C.S.; Ahmad, M.; Shibao, R. Mechanism of vasorelaxation induced by eupatorin in the rats aortic ring. Eur. J. Pharmacol. 2016, 789, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Suárez, P.A.; Navarro-Huerta, M.P.; Valle-Aguilera, J.R.; Brito-Orta, M.D.; Rodriguez-Menchaca, A.; Arechiga-Figueroa, I.; Espinosa-Tanguma, R.; Gonzalez-Chavez, M. On the mechanism of action of the relaxing effect of the 5, 4′-dihidroxi-6, 7, 8, 3′-tetrametoxi-flavone, flavone A, on vascular smooth muscle of the rat. FASEB J. 2015, 29, 773–778. [Google Scholar]
- Yorsin, S.; Sukpondma, Y.; Jansakul, C. Vasorelaxant effects of 3, 5, 7, 3′, 4′-pentamethoxyflavone isolated from Kaempferia parviflora: Partly stimulating the release of NO and H2S by rat thoracic aorta. J. Physiol. Biomed. Sci. 2015, 28, 5–14. [Google Scholar]
- Yorsin, S.; Kanokwiroon, K.; Radenahmad, N.; Jansakul, C. Increased vascular eNOS and cystathionine-γ-lyase protein after 6 weeks oral administration of 3, 5, 7, 3′, 4′-pentamethoxyflavone to middle-aged male rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Spiga, O.; Trezza, A.; Sgaragli, G.; Saponara, S. The surge of flavonoids as novel, fine regulators of cardiovascular CaV channels. Eur. J. Pharmacol. 2017, 796, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.A.; Iqbal, H.; Basir, S.F. Mechanism of flavonoids action in smooth muscle relaxation. WJPPS 2017, 6, 514–550. [Google Scholar]
- Arrieta, J.; Benitez, J.; Flores, E.; Castillo, C.; Navarrete, A. Purification of gastroprotective triterpenoids from the stem bark of Amphipterygium adstringens; role of prostaglandins, sulfhydryls, nitric oxide and capsaicin-sensitive neurons. Planta Med. 2003, 69, 905–909. [Google Scholar] [PubMed]
- García, A.; Ramírez-Apan, T.; Cogordan, J.A.; Delgado, G. Absolute configuration assignments by experimental and theoretical approaches of ent-labdane-and cis-ent-clerodane-type diterpenes isolated from Croton glabellus. Can. J. Chem. 2006, 84, 1593–1602. [Google Scholar] [CrossRef]
- Burgueño-Tapia, E.; Castillo, L.; González-Coloma, A.; Joseph-Nathan, P. Antifeedant and phytotoxic activity of the sesquiterpene p-benzoquinone perezone and some of its derivatives. J. Chem. Ecol. 2008, 34, 766. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Alvarado, C.; Rojas, A.; Mendoza, S.; Bah, M.; Gutiérrez, D.; Hernández-Sandoval, L.; Martínez, M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010, 48, 732–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galle, J.; Zabel, U.; Hübner, U.; Hatzelmann, A.; Wagner, B.; Wanner, C.; Schmidt, H.H. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br. J. Pharmacol. 1999, 127, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.; Rosas-Hernandez, H.; Jurado-Manzano, B.; Ramirez-Lee, M.A.; Salazar-Garcia, S.; Martinez-Cuevas, P.P.; Velarde-Salcedo, A.J.; Morales-Loredo, H.; Espinosa-Tanguma, R.; Ali, S.F. The prolactin family hormones regulate vascular tone through NO and prostacyclin production in isolated rat aortic rings. Acta Pharmacol. Sinica 2015, 36, 572–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Zhao, L.; Wu, R.-X.; Yue, S.-Q.; Pei, J.-M. The vasorelaxing effect of resveratrol on abdominal aorta from rats and its underlying mechanisms. Vasc. Pharmacol. 2013, 58, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Stott, J.B.; Jepps, T.A.; Greenwood, I.A. Kv7 potassium channels: A new therapeutic target in smooth muscle disorders. Drug Disc. Today 2014, 19, 413–424. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3-α-hydroxymasticadienonic acid, 3α-hydroxytirucalla-7,22Z-dien-26-oic acid, corosolic acid, galphin A, galphin B, galphimidin, 3β-trans-p-coumaroyl-oxy-16-β-hydroxy-20(29)-lupene; β-sitosteryl β-d-glucopyranoside; (3R,4R,6S)-p-menth-1-eno-3,6-diol, 6,7-diacetylaustro inulin; 6-O-acetylaustro inulin; perezon; pipitzol; 3′-demethoxy-6-O-demethyl-isoguaiacin; meso-dihydroguaiaretic acid; 5,4′-dihydroxy-3,7,8-trimethoxyflavone; 5-hydroxy-3,7,4′-trimethoxyflavone; 5,8,4´-trihydroxy-3,7-dimethoxyflavone; and pinostrobin are available from the authors. |





| Plant/Compound | EC50 (μM) | Emax (%) |
|---|---|---|
| acetilcholine (ACh) | 58.8 ± 8.9 | 69.5 ± 5.7 |
| Amphypterygium adstringens | ||
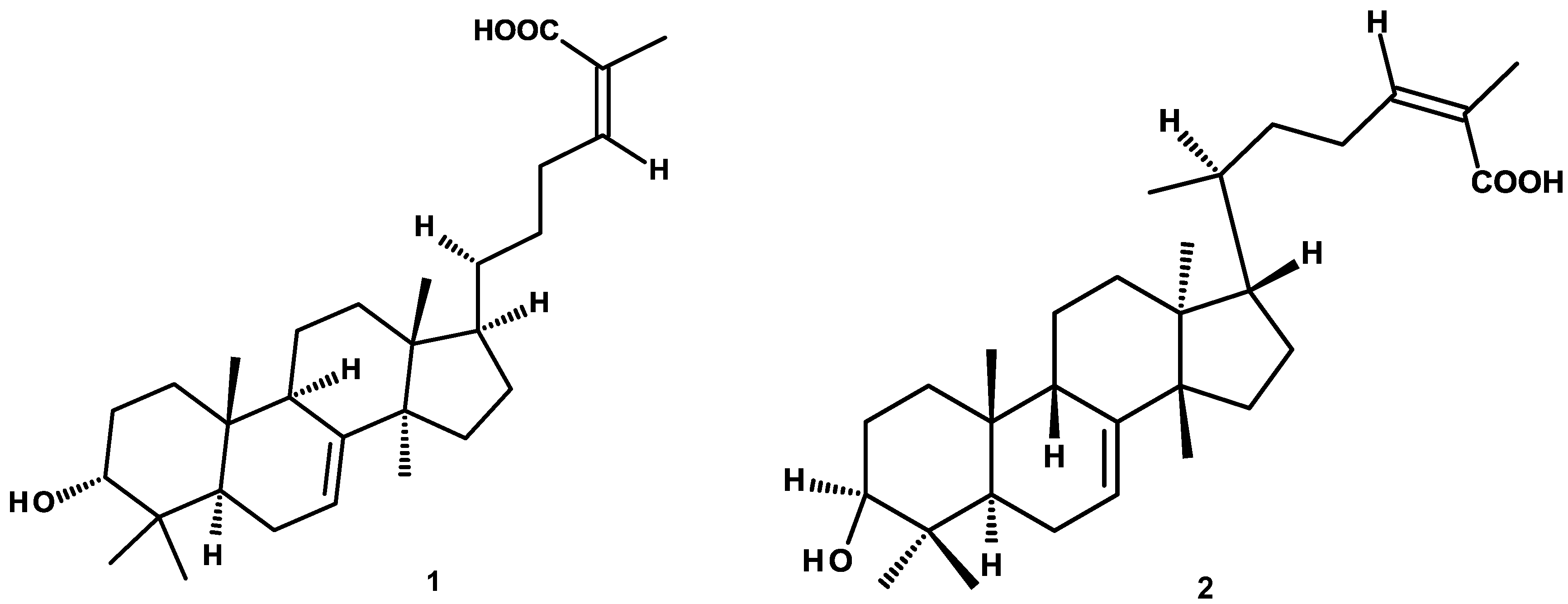
| 3-α-hydroxymasticadienonic acid (1) | 206.1 ± 11.6 | 98.2 ± 3.1 |
| Celaenodendron mexicanum | ||
| 3α-hydroxytirucalla-7,22Z-dien-26-oic acid (2) | 331.3 ± 42.1 | 99.5 ± 6.1 |
| Crataegus gracilior | ||
| Corosolic acid (3) | 108.9 ± 6.7 | 96.4 ± 4.2 |
| Croton alamosanus | ||
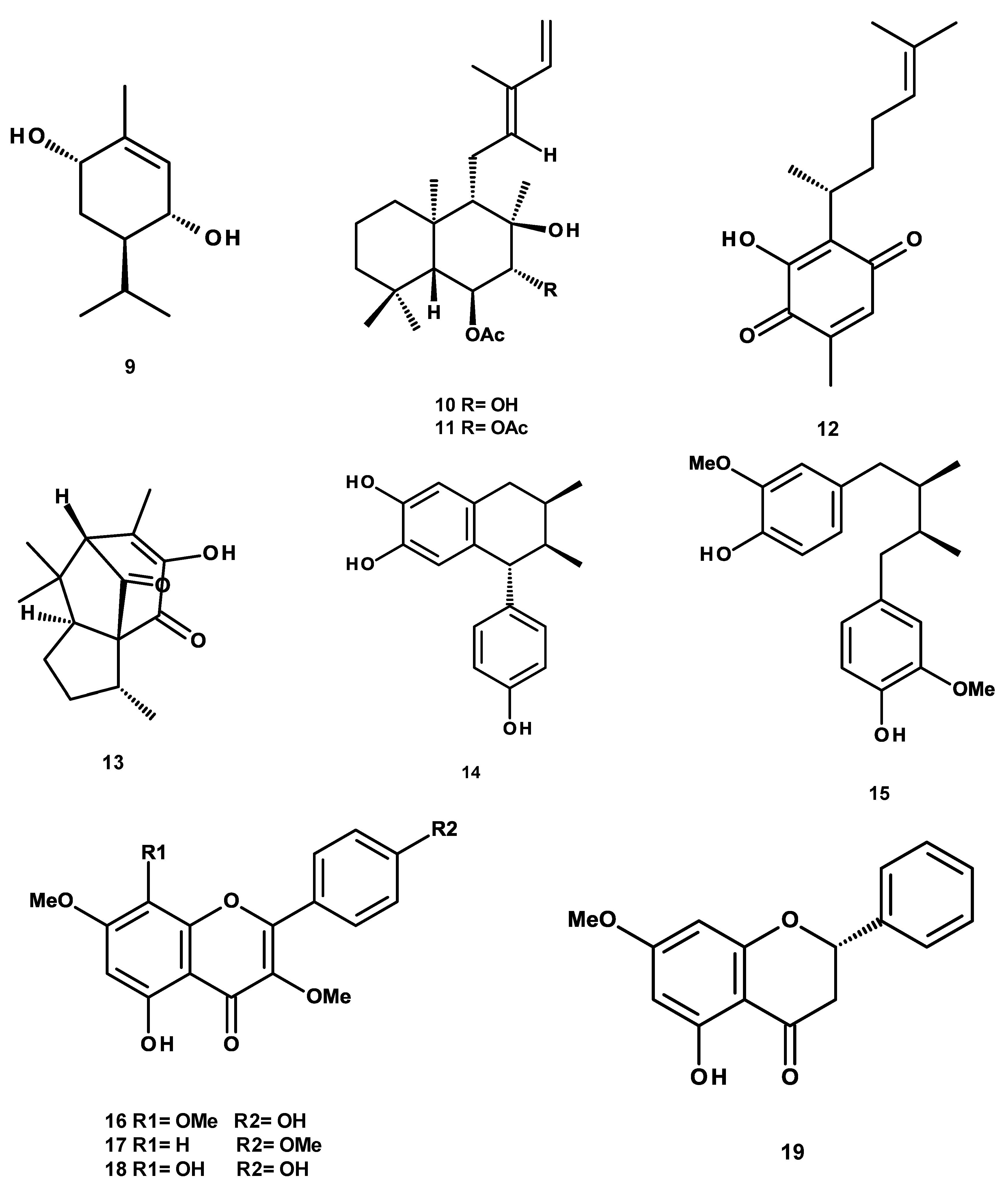
| 5-hydroxy-3,7,4′-trimethoxyflavone (17) | 377.1 ± 37.1 | 80.5 ± 3.7 |
| (3R,4R,6S)-p-menth-1-ene-3,6-diol (9) | 1622.8 ± 73.8 | 99.5 ± 8.3 |
| Croton glabellus | ||
| 6-O-acetylaustro inulin (10) | 413.5 ± 22.4 | 47.1 ± 2.8 |
| 6,7-diacetylaustro inulin (11) | 261.0 ± 9.1 | 99.5 ± 3.8 |
| Galphimia glauca | ||
| Galphin A (4) | 592.3 ± 21.7 | 99.5 ± 9.1 |
| Galphin B (5) | 1030.7 ± 39.4 | 99.5 ± 23.2 |
| Galphimidin (6) | 145.9 ± 9.2 | 99.5 ± 5.3 |
| Jatropha neopauciflora | ||
| 3β-trans-p-coumaroyl-oxy-16-β-hydroxy-20(29)-lupene (7) | 63.2 ± 5.8 | 27.5 ± 1.9 |
| β-sitosteryl β-d-glucopyranoside (8) | 314.7 ± 19.7 | 71.2 ± 5.3 |
| Larrea tridentata | ||
| meso-dihydroguaiaretic acid (15) | 49.9 ± 11.2 | 99.8 ± 2.7 |
| 5,4′-dihydroxy-3,7,8-trimethoxyflavone (16) | 587.8 ± 33.4 | 80.5 ± 5.6 |
| 5,8,4′-trihydroxy-3,7-dimethoxyflavone (18) | 122.3 ± 7.6 | 99.5 ± 5.4 |
| 3′-demethoxy-6-O-demethyl-isoguaiacin (14) | 604.5 ± 60.1 | 91.70 ± 7.3 |
| Perezia adnata | ||
| perezone (12) | 524.5 ± 32.5 | 99.5 ± 2.2 |
| pipitzol (13) | 249.4 ± 10.2 | 59.8 ± 2.4 |
| Teloxys graveolens | ||
| pinostrobin (19) | 234.9 ± 9.9 | 99.5 ± 4.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Camacho-Corona, M.D.R.; Rojas-Molina, A.; Rojas-Molina, J.I.; García, A.; Bah, M. Vasodilator Activity of Compounds Isolated from Plants Used in Mexican Traditional Medicine. Molecules 2018, 23, 1474. https://doi.org/10.3390/molecules23061474
Luna-Vázquez FJ, Ibarra-Alvarado C, Camacho-Corona MDR, Rojas-Molina A, Rojas-Molina JI, García A, Bah M. Vasodilator Activity of Compounds Isolated from Plants Used in Mexican Traditional Medicine. Molecules. 2018; 23(6):1474. https://doi.org/10.3390/molecules23061474
Chicago/Turabian StyleLuna-Vázquez, Francisco J., César Ibarra-Alvarado, María Del Rayo Camacho-Corona, Alejandra Rojas-Molina, J. Isela Rojas-Molina, Abraham García, and Moustapha Bah. 2018. "Vasodilator Activity of Compounds Isolated from Plants Used in Mexican Traditional Medicine" Molecules 23, no. 6: 1474. https://doi.org/10.3390/molecules23061474