Thermal Failure Analysis of Fiber-Reinforced Silica Aerogels under Liquid Nitrogen Thermal Shock
Abstract
:1. Introduction
2. Results and discussion
2.1. Supercritical Point Drying (SPD) and Hydrophobicity
2.2. Thermal Conductivity Failure Process
2.3. Microstructure Evolution during the Shock Processes
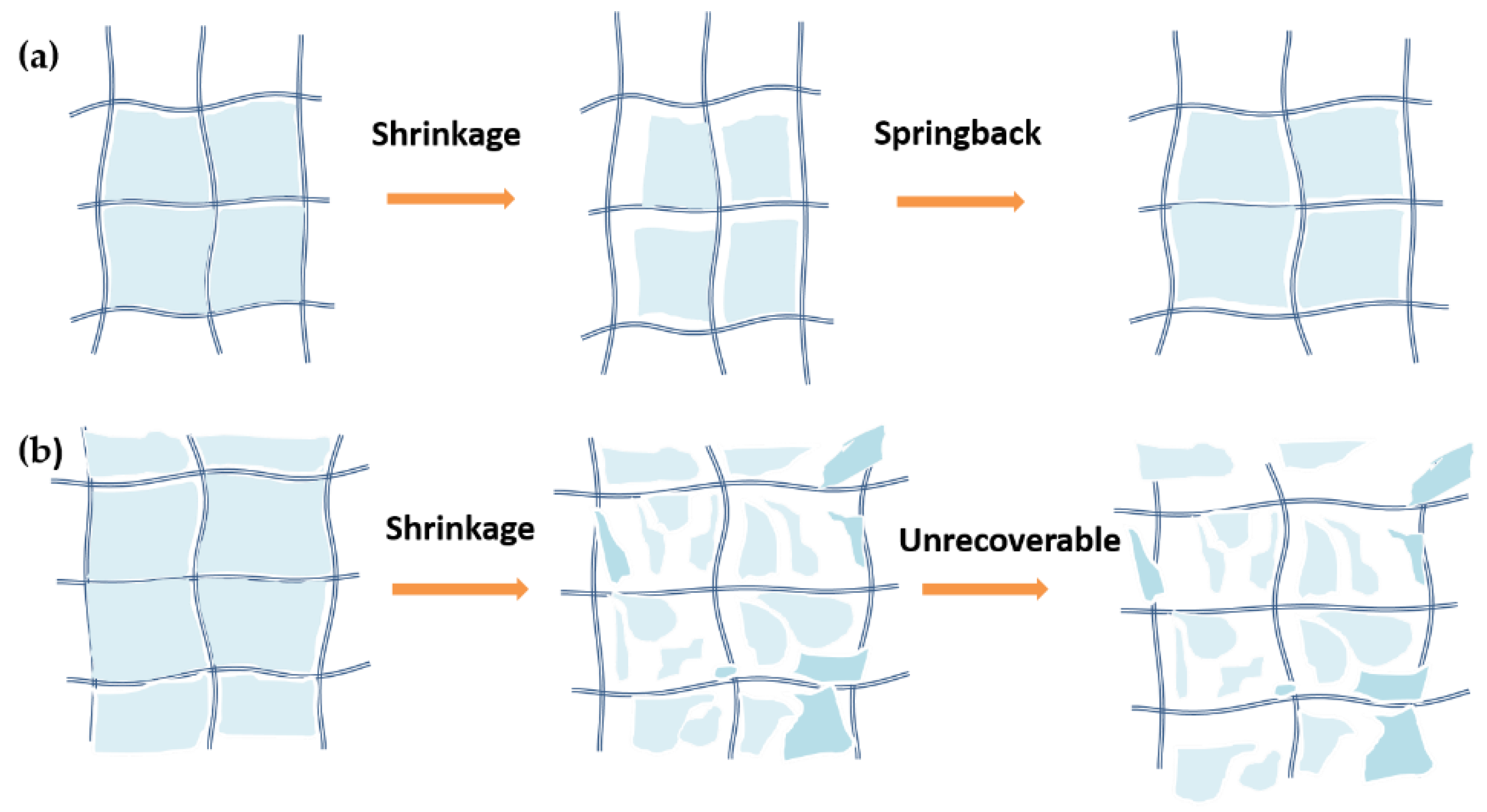
2.4. Supposed Failure Mechanism of Aerogels during the Shock Processes
3. Material and Methds
3.1. Chemicals
3.2. Synthesis of Hydrophilic and Hydrophobic Fiber-Reinforced Silica Aerogels
3.3. The Thermal Shock Experiments
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fesmire, J.; Sass, J. Aerogel insulation applications for liquid hydrogen launch vehicle tanks. Cryogenics 2008, 48, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Schmidt, M.; Schwertfeger, F. Applications for silica aerogel products. J. Non-Cryst. Solids 1998, 225, 364–368. [Google Scholar] [CrossRef]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Zhang, X.; Du, X. Radiative heat transfer study on silica aerogel and its composite insulation materials. J. Non-Cryst. Solids 2013, 362, 231–236. [Google Scholar] [CrossRef]
- Xie, T.; He, Y.-L.; Hu, Z.-J. Theoretical study on thermal conductivities of silica aerogel composite insulating material. Int. J. Heat Mass Transf. 2013, 58, 540–552. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Y.; Shi, D.; Liu, J. Experimental investigation on mechanical properties of a fiber-reinforced silica aerogel composite. Mater. Sci. Eng. A 2011, 528, 4830–4836. [Google Scholar] [CrossRef]
- Nuckols, M.; Chao, J.; Swiergosz, M. Manned Evaluation of a Prototype Composite Cold Water Diving Garment Using Liquids and Superinsulation Aerogel Materials; DTIC Document; DTIC: Fort Belvoir, VA, USA, 2005.
- Trevino, L.A.; Orndoff, E.S. Advanced Space Suit Insulation Feasibility Study; NASA: Washington, DC, USA, 2000.
- Bheekhun, N.; Abu Talib, A.R.; Hassan, M.R. Aerogels in Aerospace: An Overview. Adv. Mater. Sci. Eng. 2013, 2013, 406065. [Google Scholar] [CrossRef]
- Fricke, J.; Tillotson, T. Aerogels: Production, characterization, and applications. Thin Solid Films 1997, 297, 212–223. [Google Scholar] [CrossRef]
- Eisen, H.J.; Wen, L.; Hickey, G.; Braun, D. Sojourner Mars Rover Thermal Performance; SAE Technical Paper; SAE: Warrendale, PA, USA, 1998. [Google Scholar]
- Paul, H.L.; Diller, K.R. Comparison of thermal insulation performance of fibrous materials for the advanced space suit. J. Biomech. Eng. 2003, 125, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.S.; Phillips, C.J.; Birur, G.C.; Sunada, E.T.; Pauken, M.T. Development of a thermal control architecture for the Mars Exploration Rovers. Expand. Front. Space 2003, 654, 194–205. [Google Scholar]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.H.; Rawashdeh, A.M.M. Nanoengineering strong silica aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, J.; Wu, A.; Shen, J.; Zhou, B. High strength SiO2 aerogel insulation. J. Non-Cryst. Solids 1998, 225, 101–104. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Zhang, X.; Yu, F.; Du, X. Thermal conductivities study on silica aerogel and its composite insulation materials. Int. J. Heat Mass Transf. 2011, 54, 2355–2366. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, H.; Ding, Y.; Yin, S.; Wang, M.; Cao, A. Engineering thermal and mechanical properties of flexible fiber-reinforced aerogel composites. J. Sol-Gel Sci. Technol. 2012, 63, 445–456. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Duan, Y.-Y.; Wang, X.-D.; Wang, B.-X. Radiative properties and heat transfer characteristics of fiber-loaded silica aerogel composites for thermal insulation. Int. J. Heat Mass Transf. 2012, 55, 5196–5204. [Google Scholar] [CrossRef]
- Tillotson, T.; Hrubesh, L. Transparent ultralow-density silica aerogels prepared by a two-step sol-gel process. J. Non-Cryst. Solids 1992, 145, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zhou, B.; Wu, G.-M.; Xu, X.; Xie, D.-B.; Xu, Z.; Wang, X.-L.; Wu, Y.-H.; Shen, J. Preparation and molding of ultralow-density silica aerogels. High Power Laser Part. Beams 2005, 17, 1674–1678. [Google Scholar]
- Lee, S.; Cha, Y.C.; Hwang, H.J.; Moon, J.-W.; Han, I.S. The effect of pH on the physicochemical properties of silica aerogels prepared by an ambient pressure drying method. Mater. Lett. 2007, 61, 3130–3133. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.D.; Rao, A.V. Surface chemical modification of TEOS based silica aerogels synthesized by two step (acid–base) sol–gel process. Appl. Surf. Sci. 2006, 252, 4289–4297. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R″ Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Hegde, N.D.; Shewale, P.M. Imperviousness of the hydrophobic silica aerogels against various solvents and acids. Appl. Surf. Sci. 2007, 253, 4137–4141. [Google Scholar] [CrossRef]
- Folgar, C.; Folz, D.; Suchicital, C.; Clark, D. Microstructural evolution in silica aerogel. J. Non-Cryst. Solids 2007, 353, 1483–1490. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Z.; Sèbe, G.; Wu, R.; Rivera Virtudazo, R.V.; Tingaut, P.; Koebel, M.M. Multiscale Assembly of Superinsulating Silica Aerogels Within Silylated Nanocellulosic Scaffolds: Improved Mechanical Properties Promoted by Nanoscale Chemical Compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Schwan, M.; Ratke, L. Flexibilisation of resorcinol–formaldehyde aerogels. J. Mater. Chem. A 2013, 1, 13462–13468. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Stadermann, M.; Shin, S.J.; Satcher, J.H., Jr.; Gammon, S.A.; Letts, S.A.; van Buuren, T.; Hamza, A.V. Super-compressibility of ultralow-density nanoporous silica. Adv. Mater. 2012, 24, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Scheuerpflug, P.; Morper, H.-J.; Neubert, G. Low-temperature thermal transport in silica aerogels. J. Phys. D Appl. Phys. 1991, 24, 1395. [Google Scholar] [CrossRef]
- Bangi, U.K.H.; Venkateswara Rao, A.; Parvathy Rao, A. A new route for preparation of sodium-silicate-based hydrophobic silica aerogels via ambient-pressure drying. Sci. Technol. Adv. Mater. 2016, 9, 035006. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Jung, H.-H.; Hyun, S.-H.; Ahn, Y.-S. Effective preparation of crack-free silica aerogels via ambient drying. J. Sol-Gel Sci. Technol. 2007, 41, 139–146. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



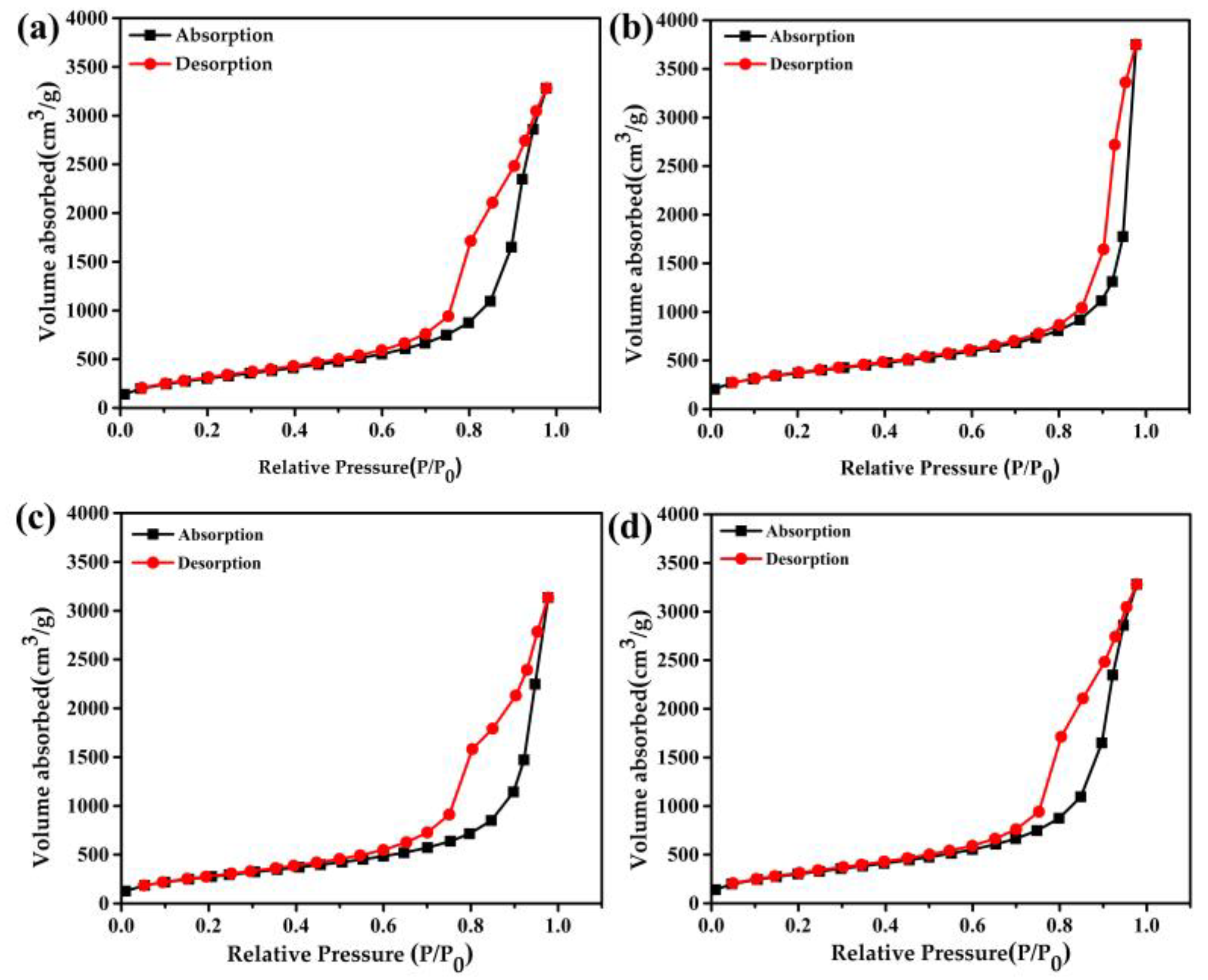




| Sample | Density (mg/cm3) | Specific Surface Area (m2/g) | Average Pore Size (nm) | Peak Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|---|
| a | 140 | 1521 | 16.3 | 18.0 | 6.1 |
| b | 1316 | 17.7 | 24.9 | 5.8 | |
| c | 150 | 1002 | 19.4 | 9.7 | 4.9 |
| d | 1117 | 18.2 | 9.7 | 5.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, A.; Liu, M.; Huang, S.; Li, C.; Zhou, B. Thermal Failure Analysis of Fiber-Reinforced Silica Aerogels under Liquid Nitrogen Thermal Shock. Molecules 2018, 23, 1522. https://doi.org/10.3390/molecules23071522
Du A, Liu M, Huang S, Li C, Zhou B. Thermal Failure Analysis of Fiber-Reinforced Silica Aerogels under Liquid Nitrogen Thermal Shock. Molecules. 2018; 23(7):1522. https://doi.org/10.3390/molecules23071522
Chicago/Turabian StyleDu, Ai, Mingfang Liu, Shangming Huang, Conghang Li, and Bin Zhou. 2018. "Thermal Failure Analysis of Fiber-Reinforced Silica Aerogels under Liquid Nitrogen Thermal Shock" Molecules 23, no. 7: 1522. https://doi.org/10.3390/molecules23071522




