Nickel Sulfides Decorated SiC Foam for the Low Temperature Conversion of H2S into Elemental Sulfur
Abstract
:1. Introduction
2. Experimental Section
2.1. General Procedure for the Preparation of NiS2-Decorated SiC; Materials and Methods
2.2. Selective Oxidation of H2S to Elemental Sulfur
2.3. Characterization Techniques
3. Results and Discussion
3.1. NiS2/SiC Synthesis and Characterization
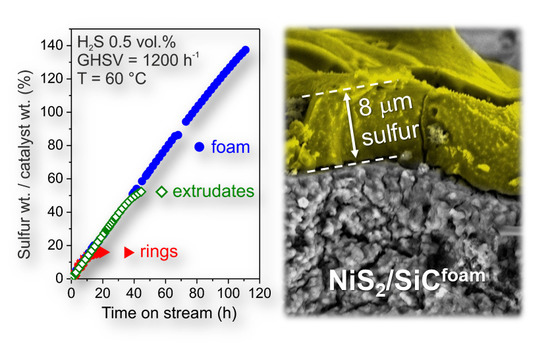
3.2. Desulfurization Process
3.2.1. Influence of SiC Support on the Desulfurization Performance
3.2.2. Catalyst Regeneration and H2S Concentration Effects
3.2.3. Influence of the Reaction Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Chou, S.J. Hydrogen Sulfide: Human Health Aspects; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Zhang, X.; Tang, Y.Y.; Qu, S.Q.; Da, J.W.; Hao, Z.P. H2S-selective catalytic oxidation: Catalysts and processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Wiheeb, A.D.; Shamsudin, I.K.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M.R. Present technologies for hydrogen sulfide removal from gaseous mixtures. Rev. Chem. Eng. 2013, 29, 449–470. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Desulfurization of digester gas on catalytic carbonaceous adsorbents: Complexity of interactions between the surface and components of the gaseous mixture. Ind. Eng. Chem. Res. 2006, 45, 3658–3665. [Google Scholar] [CrossRef]
- Pieplu, A.; Saur, O.; Lavalley, J.C.; Legendre, O.; Nedez, C. Claus catalysis and H2S selective oxidation. Catal. Rev. Sci. Eng. 1998, 40, 409–450. [Google Scholar] [CrossRef]
- Wiȩckowska, J. Catalytic and adsorptive desulphurization of gases. Catal. Today 1995, 24, 405–465. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Truong-Phuoc, L.; Tran-Thanh, T.; Nhut, J.M.; Nguyen-Dinh, L.; Janowska, I.; Begin, D.; Pham-Huu, C. Nitrogen-doped carbon nanotubes decorated silicon carbide as a metal-free catalyst for partial oxidation of H2S. Appl. Catal. A Gen. 2014, 482, 397–406. [Google Scholar]
- Keller, N.; Pham-Huu, C.; Estornes, C.; Ledoux, M.J. Low temperature use of SiC-supported NiS2-based catalysts for selective H2S oxidation—Role of SiC surface heterogeneity and nature of the active phase. Appl. Catal. A Gen. 2002, 234, 191–205. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Pham-Huu, C.; Keller, N.; Nougayrede, J.B.; Savin-Poncet, S.; Bousquet, J. Selective oxidation of H2S in claus tail-gas over SiC supported NiS2 catalyst. Catal. Today 2000, 61, 157–163. [Google Scholar] [CrossRef]
- Primavera, A.; Trovarelli, A.; Andreussi, P.; Dolcetti, G. The effect of water in the low-temperature catalytic oxidation of hydrogen sulfide to sulfur over activated carbon. Appl. Catal. A Gen. 1998, 173, 185–192. [Google Scholar] [CrossRef]
- Sun, F.G.; Liu, J.; Chen, H.C.; Zhang, Z.X.; Qiao, W.M.; Long, D.H.; Ling, L.C. Nitrogen-rich mesoporous carbons: Highly efficient, regenerable metal-free catalysts for low-temperature oxidation of H2S. ACS Catal. 2013, 3, 862–870. [Google Scholar] [CrossRef]
- Twigg, M.V.; Richardson, J.T. Theory and applications of ceramic foam catalysts. Chem. Eng. Res. Des. 2002, 80, 183–189. [Google Scholar] [CrossRef]
- Tschentscher, R.; Spijkers, R.J.P.; Nijhuis, T.A.; van der Schaaf, J.; Schouten, J.C. Liquid-solid mass transfer in agitated slurry reactors and rotating solid foam reactors. Ind. Eng. Chem. Res. 2010, 49, 10758–10766. [Google Scholar] [CrossRef]
- Leon, M.A.; Tschentscher, R.; Nijhuis, T.A.; van der Schaaf, J.; Schouten, J.C. Rotating foam stirrer reactor: Effect of catalyst coating characteristics on reactor performance. Ind. Eng. Chem. Res. 2011, 50, 3184–3193. [Google Scholar] [CrossRef]
- Leon, M.A.; Nijhuis, T.A.; van der Schaaf, J.; Schouten, J.C. Mass transfer modeling of a consecutive reaction in rotating foam stirrer reactors: Selective hydrogenation of a functionalized alkyne. Chem. Eng. Sci. 2012, 73, 412–420. [Google Scholar] [CrossRef]
- Tschentscher, R.; Nijhuis, T.A.; van der Schaaf, J.; Schouten, J.C. Glucose oxidation in slurry reactors and rotating foam reactors. Ind. Eng. Chem. Res. 2012, 51, 1620–1634. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Hantzer, S.; Pham-Huu, C.; Guille, J.; Desaneaux, M.P. New synthesis and uses of high-specific-surface SiC as a catalytic support that is chemically inert and has high thermal-resistance. J. Catal. 1988, 114, 176–185. [Google Scholar] [CrossRef]
- Nguyen, P.; Pham, C. Innovative porous SiC-based materials: From nanoscopic understandings to tunable carriers serving catalytic needs. Appl. Catal. A Gen. 2011, 391, 443–454. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Ba, H.; El-Berrichi, Z.; Nhut, J.M.; Ledoux, M.J.; Liu, Y.F.; Pham-Huu, C. Silicon carbide foam as a porous support platform for catalytic applications. New J. Chem. 2016, 40, 4285–4299. [Google Scholar] [CrossRef]
- Lacroix, M.; Dreibine, L.; de Tymowski, B.; Vigneron, F.; Edouard, D.; Begin, D.; Nguyen, P.; Pham, C.; Savin-Poncet, S.; Luck, F.; et al. Silicon carbide foam composite containing cobalt as a highly selective and re-usable Fischer-Tropsch synthesis catalyst. Appl. Catal. A Gen. 2011, 397, 62–72. [Google Scholar] [CrossRef]
- Chizari, K.; Deneuve, A.; Ersen, O.; Florea, I.; Liu, Y.; Edouard, D.; Janowska, I.; Begin, D.; Pham-Huu, C. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for selective oxidation. ChemSusChem 2012, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Truong-Phuoc, L.; Tri, T.H.; Lam, N.D.; Baaziz, W.; Romero, T.; Edouard, D.; Begin, D.; Janowska, I.; Pham-Huu, C. Silicon carbide foam decorated with carbon nanofibers as catalytic stirrer in liquid-phase hydrogenation reactions. Appl. Catal. A Gen. 2014, 469, 81–88. [Google Scholar] [CrossRef]
- Fernandez, A.; Arzac, G.M.; Vogt, U.F.; Hosoglu, F.; Borgschulte, A.; de Haro, M.C.J.; Montes, O.; Zuttel, A. Investigation of a Pt containing washcoat on SiC foam for hydrogen combustion applications. Appl. Catal. B Environ. 2016, 180, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Ou, X.; Xu, S.; Warnett, J.M.; Holmes, S.M.; Zaheer, A.; Garforth, A.A.; Williams, M.A.; Jiao, Y.; Fan, X. Creating hierarchies promptly: Microwave-accelerated synthesis of zsm-5 zeolites on macrocellular silicon carbide (SiC) foams. Chem. Eng. J. 2017, 312, 1–9. [Google Scholar] [CrossRef]
- Ahmed, J.; Pham-Huu, C.; Edouard, D. A predictive model based on tortuosity for pressure drop estimation in ‘slim’ and ‘fat’ foams. Chem. Eng. Sci. 2011, 66, 4771–4779. [Google Scholar] [CrossRef]
- Edouard, D.; Ivanova, S.; Lacroix, M.; Vanhaecke, E.; Pham, C.; Pham-Huu, C. Pressure drop measurements and hydrodynamic model description of SiC foam composites decorated with SiC nanofiber. Catal. Today 2009, 141, 403–408. [Google Scholar] [CrossRef]
- Ba, H.; Liu, Y.; Mu, X.; Doh, W.-H.; Nhut, J.-M.; Granger, P.; Pham-Huu, C. Macroscopic nanodiamonds/β-SiC composite as metal-free catalysts for steam-free dehydrogenation of ethylbenzene to styrene. Appl. Catal. A Gen. 2015, 499, 217–226. [Google Scholar] [CrossRef]
- Sternmet, C.P.; Meeuwse, A.; van der Schaaf, J.; Kuster, B.F.M.; Schouten, J.C. Gas-liquid mass transfer and axial dispersion in solid foam packings. Chem. Eng. Sci. 2007, 62, 5444–5450. [Google Scholar]
- Lacroix, M.; Nguyen, P.; Schweich, D.; Pham-Huu, C.; Savin-Poncet, S.; Edouard, D. Pressure drop measurements and modeling on SiC foams. Chem. Eng. Sci. 2007, 62, 3259–3267. [Google Scholar] [CrossRef]
- Liu, Y.; Podila, S.; Nguyen, D.L.; Edouard, D.; Nguyen, P.; Pham, C.; Ledoux, M.J.; Pham-Huu, C. Methanol dehydration to dimethyl ether in a platelet milli-reactor filled with H-ZSM5/SiC foam catalyst. Appl. Catal. A Gen. 2011, 409, 113–121. [Google Scholar] [CrossRef]
- Liu, Y.; Edouard, D.; Nguyen, L.D.; Begin, D.; Nguyen, P.; Pham, C.; Cuong, P.H. High performance structured platelet milli-reactor filled with supported cobalt open cell SiC foam catalyst for the Fischer-Tropsch synthesis. Chem. Eng. J. 2013, 222, 265–273. [Google Scholar] [CrossRef]
- Ba, H.; Luo, J.; Liu, Y.; Duong-Viet, C.; Tuci, G.; Giambastiani, G.; Nhut, J.-M.; Nguyen-Dinh, L.; Ersen, O.; Su, D.S. Macroscopically shaped monolith of nanodiamonds @nitrogen-enriched mesoporous carbon decorated SiC as a superior metal-free catalyst for the styrene production. Appl. Catal. B Environ. 2017, 200, 343–350. [Google Scholar] [CrossRef]
- Deneuve, A.; Florea, I.; Ersen, O.; Nguyen, P.; Pham, C.; Begin, D.; Edouard, D.; Ledoux, M.J.; Pham-Huu, C. Catalytic growth of silicon carbide composite with nanoscopic properties and enhanced oxidative resistance as catalyst support. Appl. Catal. A Gen. 2010, 385, 52–61. [Google Scholar] [CrossRef]
- Fan, X.L.; Ou, X.X.; Xing, F.; Turley, G.A.; Denissenko, P.; Williams, M.A.; Batail, N.; Pham, C.; Lapkin, A.A. Microtomography-based numerical simulations of heat transfer and fluid flow through beta-sic open-cell foams for catalysis. Catal. Today 2016, 278, 350–360. [Google Scholar] [CrossRef]
- General scheme for the Sulfidation process: MOX(s) + H2S(g) = MSX(s) + xH2O(g). See also: Christoforou, S.C.; Efthimiadis, E.A.; Vasalos, I.A. Sulfidation of mixed metal oxides in a fluidized-bed reactor. Ind. Eng. Chem. Res. 1995, 34, 83–93. [Google Scholar] [CrossRef]
- Eck, J.; Balat-Pichelin, M.; Charpentier, L.; Bêche, E.; Audubert, F. Behavior of SiC at high temperature under helium with low oxygen partial pressure. J. Eur. Ceram. Soc. 2008, 28, 2995–3004. [Google Scholar] [CrossRef]
- Keller, N.; Di Grégorio, F.; Pham-Huu, C.; Keller, V. Towards the oxygenated phase coverage rate of β-SiC surface. Diam. Relat. Mater. 2008, 17, 1867–1870. [Google Scholar] [CrossRef]
- Shimoda, K.; Park, J.S.; Hinoki, T.; Kohyama, A. Influence of surface structure of SiC nano-sized powder analyzed by X-ray photoelectron spectroscopy on basic powder characteristics. Appl. Surf. Sci. 2007, 253, 9450–9456. [Google Scholar] [CrossRef]
- Gavrikov, A.; Knizhnik, A.; Safonov, A.; Scherbinin, A.; Bagatur’yants, A.; Potapkin, B.; Chatterjee, A.; Matocha, K. First-principles-based investigation of kinetic mechanism of SiC(0001) dry oxidation including defect generation and passivation. J. Appl. Phys. 2008, 104, 093508. [Google Scholar] [CrossRef]
- Severino, A.; Camarda, M.; Scalese, S.; Fiorenza, P.; Di Franco, S.; Bongiorno, C.; La Magna, A.; La Via, F. Preferential oxidation of stacking faults in epitaxial off-axis (111) 3 C-SiC films. Appl. Phys. Lett. 2009, 95, 111905. [Google Scholar] [CrossRef]
- Chowdhury, A.I.; Tollefson, E.L. Catalyst modification and process design considerations for the oxidation of low concentrations of hydrogen-sulfide in natural-gas. Can. J. Chem. Eng. 1990, 68, 449–454. [Google Scholar] [CrossRef]
- Dalai, A.K.; Majumdar, A.; Chowdhury, A.; Tollefson, E.L. The effects of pressure and temperature on the catalytic-oxidation of hydrogen-sulfide in natural-gas and regeneration of the catalyst to recover the sulfur produced. Can. J. Chem. Eng. 1993, 71, 75–82. [Google Scholar] [CrossRef]
- Liu, Y.F.; de Tymowski, B.; Vigneron, F.; Florea, I.; Ersen, O.; Meny, C.; Nguyen, P.; Pham, C.; Luck, F.; Pham-Huu, C. Titania-decorated silicon carbide-containing cobalt catalyst for fischer-tropsch synthesis. ACS Catal. 2013, 3, 393–404. [Google Scholar] [CrossRef]
- Elamin, M.M.; Muraza, O.; Malaibari, Z.; Ba, H.; Nhut, J.M.; Pham-Huu, C. Microwave assisted growth of SAPO-34 on b-SiC foams for methanol dehydration to dimethyl ether. Chem. Eng. J. 2015, 274, 113–122. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Jiang, C.H.; Yang, Z.M.; Zhang, J.S. Controllable synthesis of ZSM-5 coatings on SiC foam support for mtp application. Microporous Mesoporous Mater. 2012, 162, 152–158. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Jiang, C.H.; Yang, Z.M.; Liu, J.; Zhang, J.S. Synthesis of highly accessible ZSM-5 coatings on SiC foam support for MTP reaction. Microporous Mesoporous Mater. 2013, 181, 201–207. [Google Scholar] [CrossRef]
- Klein, J.; Henning, K.-D. Catalytic oxidation of hydrogen sulphide on activated carbons. Fuel 1984, 63, 1064–1067. [Google Scholar] [CrossRef]
- Florea, I.; Ersen, O.; Hirlimann, C.; Roiban, L.; Deneuve, A.; Houlle, M.; Janowska, I.; Nguyen, P.; Pham, C.; Pham-Huu, C. Analytical electron tomography mapping of the SiC pore oxidation at the nanoscale. Nanoscale 2010, 2, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Steijns, M.; Derks, F.; Verloop, A.; Mars, P. The mechanism of the catalytic oxidation of hydrogen sulfide. J. Catal. 1976, 42, 87–95. [Google Scholar] [CrossRef]
Sample Availability: Sample of compounds NiS2/SiCfoam is available from the authors on request. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong-Viet, C.; Nguyen-Dinh, L.; Liu, Y.; Tuci, G.; Giambastiani, G.; Pham-Huu, C. Nickel Sulfides Decorated SiC Foam for the Low Temperature Conversion of H2S into Elemental Sulfur. Molecules 2018, 23, 1528. https://doi.org/10.3390/molecules23071528
Duong-Viet C, Nguyen-Dinh L, Liu Y, Tuci G, Giambastiani G, Pham-Huu C. Nickel Sulfides Decorated SiC Foam for the Low Temperature Conversion of H2S into Elemental Sulfur. Molecules. 2018; 23(7):1528. https://doi.org/10.3390/molecules23071528
Chicago/Turabian StyleDuong-Viet, Cuong, Lam Nguyen-Dinh, Yuefeng Liu, Giulia Tuci, Giuliano Giambastiani, and Cuong Pham-Huu. 2018. "Nickel Sulfides Decorated SiC Foam for the Low Temperature Conversion of H2S into Elemental Sulfur" Molecules 23, no. 7: 1528. https://doi.org/10.3390/molecules23071528