Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
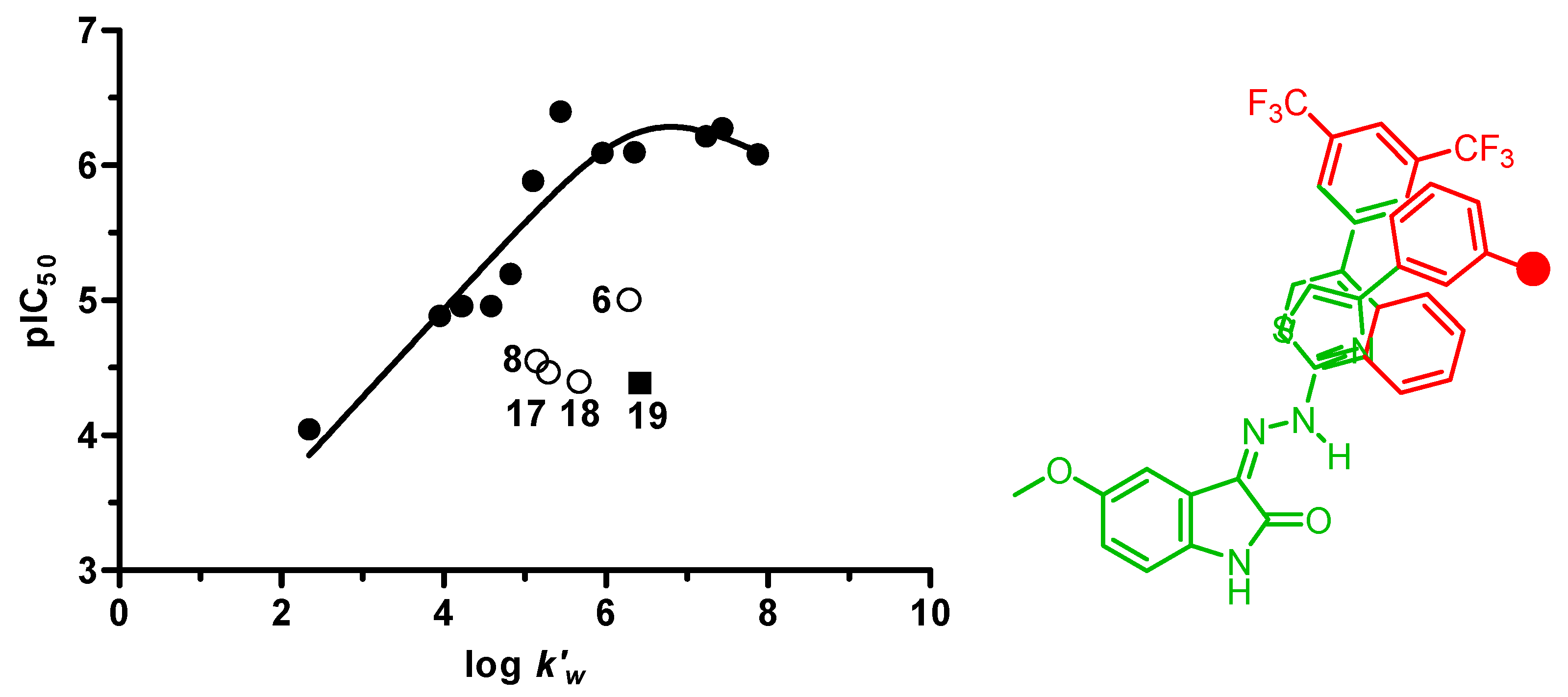
2.2. Inhibition of Amyloid Aggregation
n = 13, r2 = 0.900, s = 0.271, logβ = −6.47, optimum log k’w = 6.80
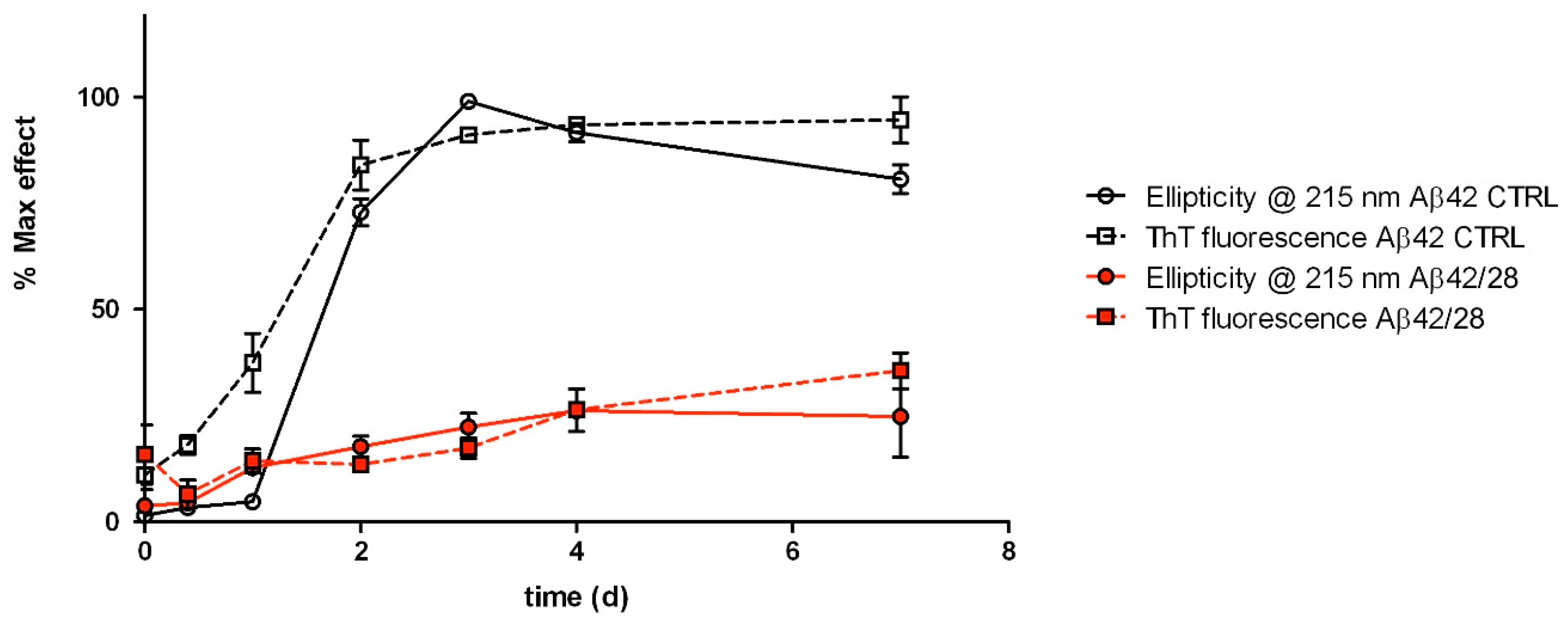
2.3. Kinetics of Aβ42 Fibrillization
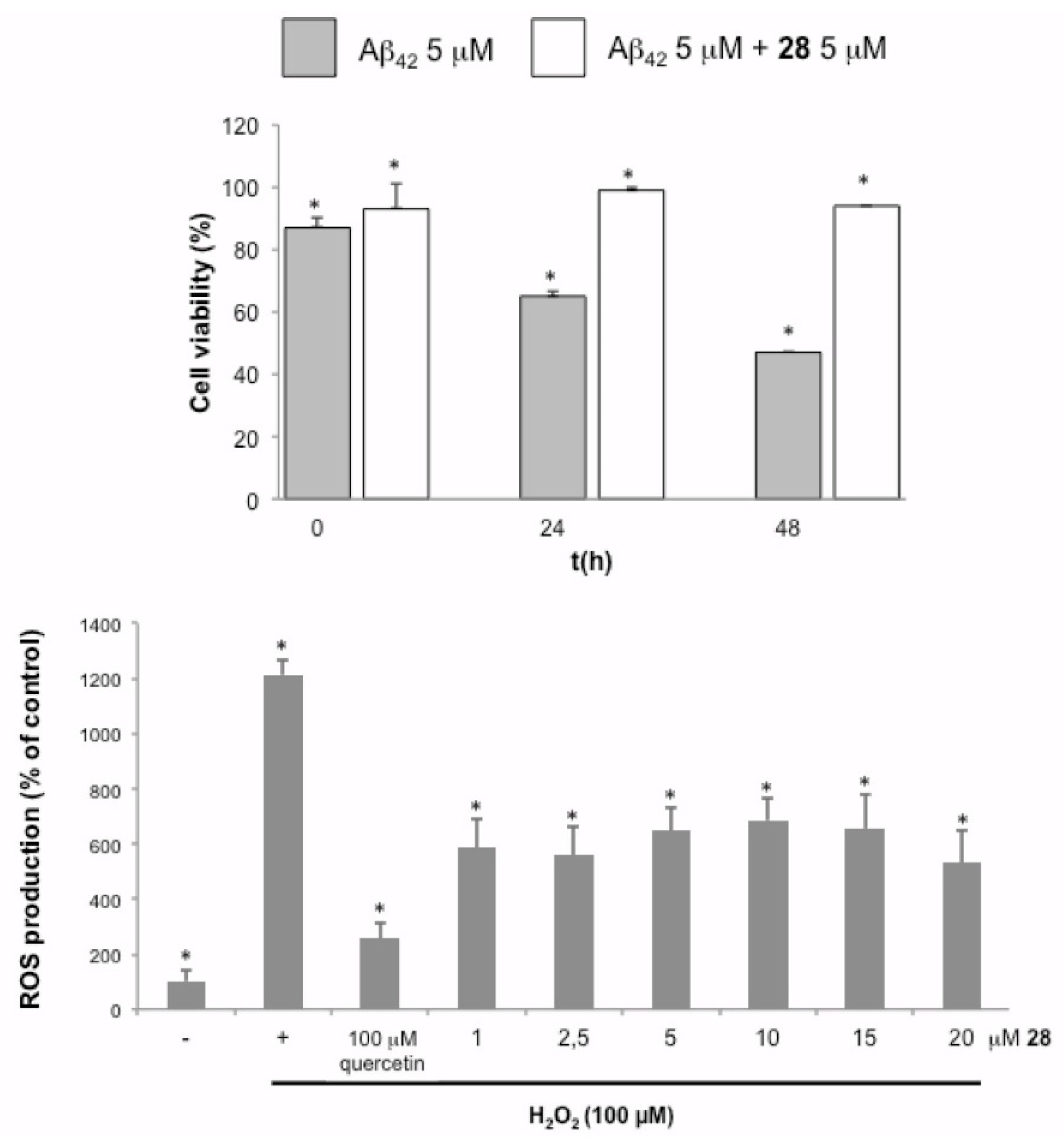
2.4. Protection Assays Against Oxidative and Cytotoxic Effects
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 5-methoxy-1H-indol-2,3-dione 3-arylhydrazones 4–7, 9 and 11
3.1.2. Synthesis of 3-arylhydrazono-indolin-2-ones 8 and 10
3.1.3. Synthesis of 5-Methoxy-3-(2-thiazolylhydrazono)indolin-2-ones 13–18
3.1.4. Synthesis of Compound 19
3.1.5. Synthesis of 3-(Benzylidenehydrazono)-5-methoxyindolin-2-ones 21 and 22
3.1.6. Synthesis of Carboxyhydrazide 23
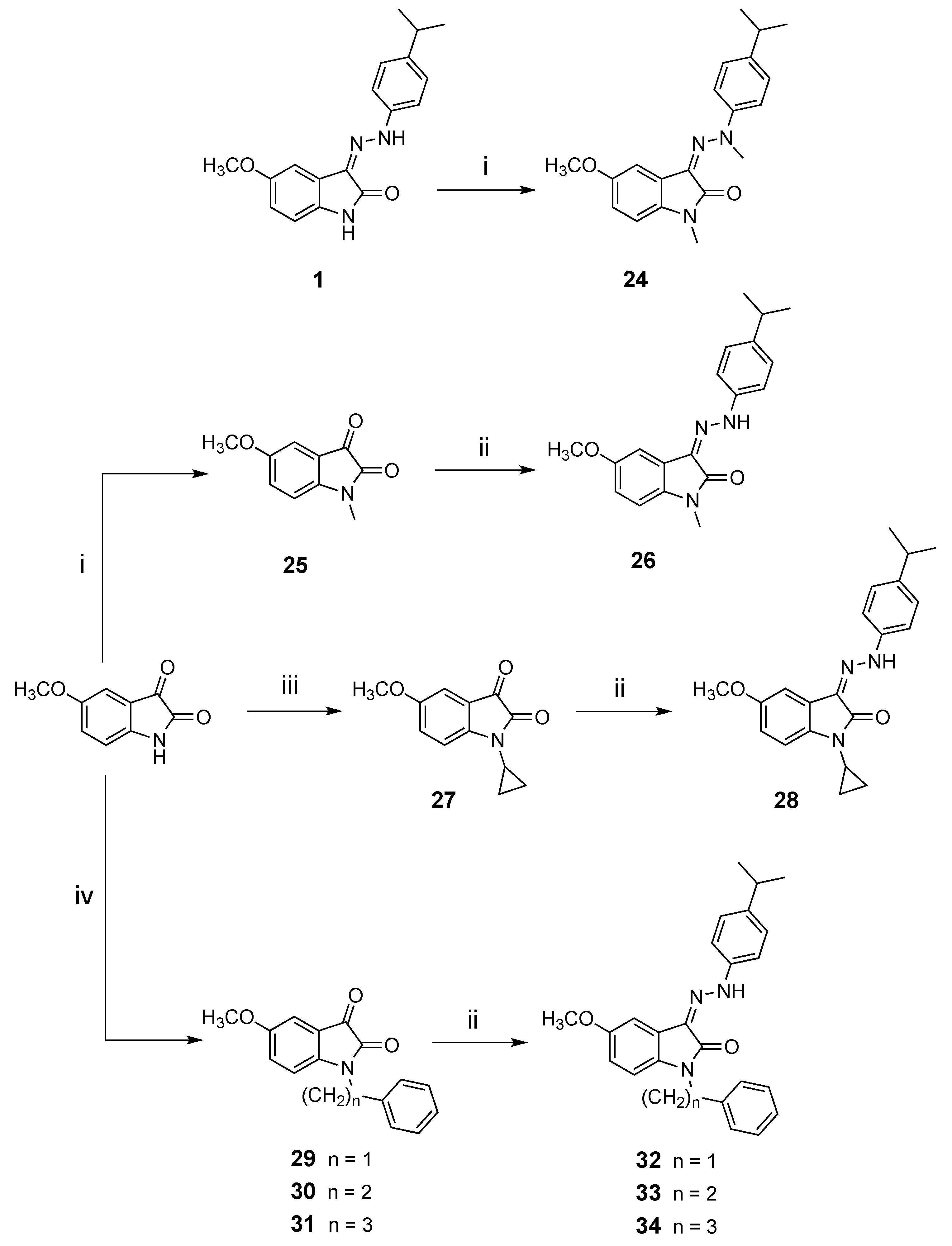
3.1.7. Synthesis of N-alkylisatin Derivatives 24, 30 and 31
3.1.8. Synthesis of N-cyclopropyl-5-methoxyindolin-2,3-dione (27)
3.1.9. Synthesis of N-alkyl-3-phenylhyhydrazonoindolin-2-ones 26, 28, 32–34
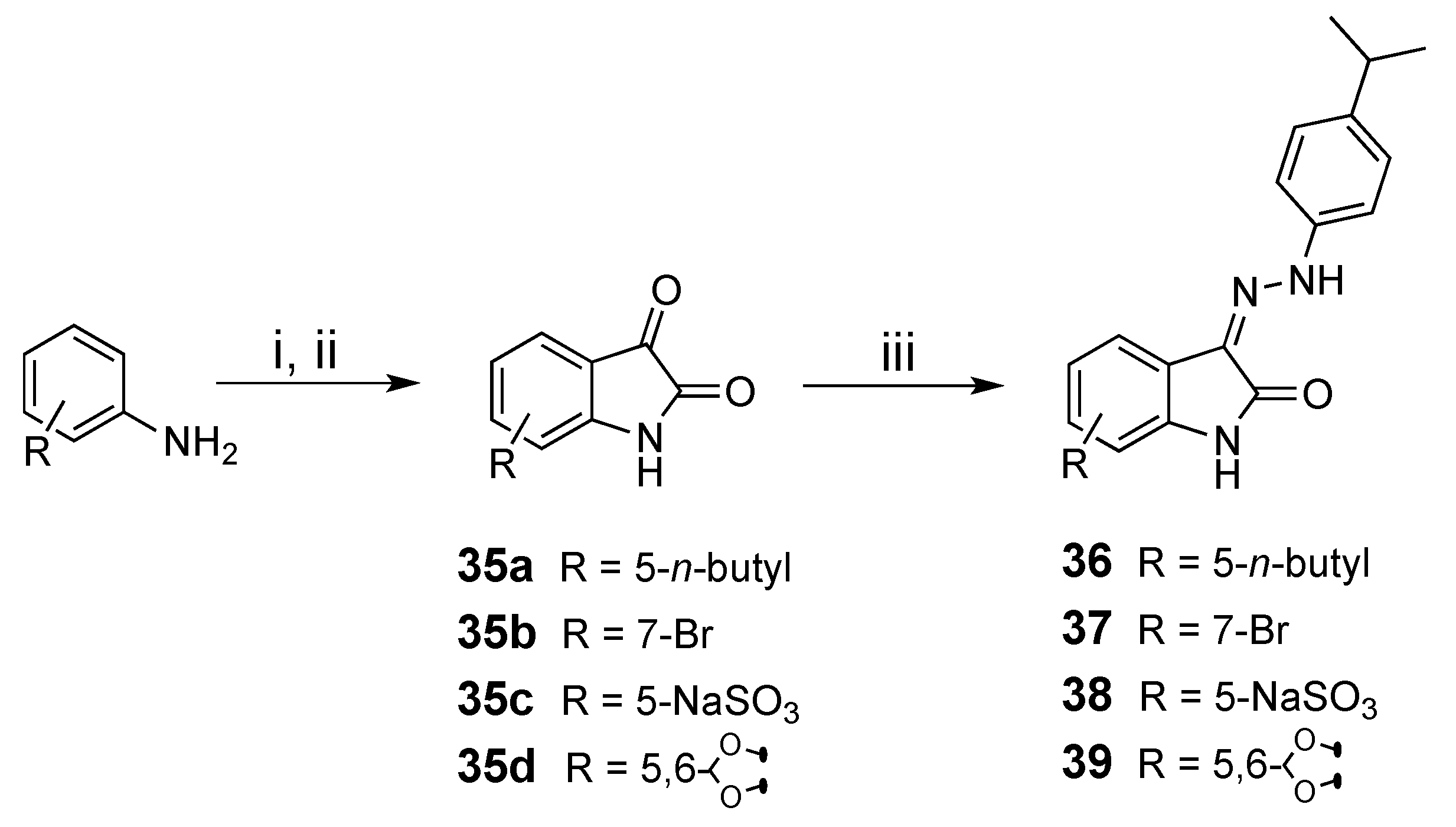
3.1.10. Synthesis of 4-isopropylphenylhydrazonoindolin-2-ones 36–39
3.2. Determination of Llipophilicity by RP-HPLC
3.3. Inhibition of Aβ40 Aggregation
3.4. Cell-Based Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2016 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar]
- Anand, P.; Singh, B. A Review on Cholinesterase Inhibitors for Alzheimer’s Disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.; Grossberg, G.T. Use of Memantine for the Treatment of Dementia. Expert Rev. Neurother. 2011, 11, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Mortsdorf, T.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dement. (N. Y.) 2017, 3, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Levine, H., III. Small molecule inhibitors of Abeta assembly. Amyloid 2007, 14, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Egea, J.; de Los Ríos, C.; Marco-Contelles, J.; Romero, A. Melatonin as a versatile molecule to design novel multitarget hybrids against neurodegeneration. Future Med. Chem. 2017, 9, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Török, M.; Abid, M.; Mhadgut, S.C.; Török, B. Organofluorine inhibitors of amyloid fibrillogenesis. Biochemistry 2006, 45, 5377–5383. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Frydman-Marom, A.; Rechter, M.; Gazit, E. Inhibition of amyloid fibril formation and cytotoxicity by hydroxyindole derivatives. Biochemistry 2006, 45, 4727–4735. [Google Scholar] [CrossRef] [PubMed]
- Gsponer, J.; Haberthur, U.; Caflisch, A. The role of side-chain interactions in the early steps of aggregation: Molecular dynamics simulations of an amyloid-forming peptide from the yeast prion Sup35. Proc. Natl. Acad. Sci. USA 2003, 100, 5154–5159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hills, R.D., Jr.; Brooks, C.L., III. Hydrophobic cooperativity as a mechanism for amyloid nucleation. J. Mol. Biol. 2007, 368, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Convertino, M.; Pellarin, R.; Catto, M.; Carotti, A.; Caflisch, A. 9,10-Anthraquinone hinders beta-aggregation: How does a small molecule interfere with Abeta-peptide amyloid fibrillation? Protein Sci. 2009, 18, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ma, B.; Nussinov, R. Consensus features in amyloid fibrils: Sheet-sheet recognition via a (polar or nonpolar) zipper structure. Phys. Biol. 2006, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cellamare, S.; Stefanachi, A.; Stolfa, D.A.; Basile, T.; Catto, M.; Campagna, F.; Sotelo, E.; Acquafredda, P.; Carotti, A. Design, Synthesis, and Biological Evaluation of Glycine-based Molecular Tongs as Inhibitors of Abeta1-40 Aggregation in Vitro. Bioorg. Med. Chem. 2008, 16, 4810–4822. [Google Scholar] [CrossRef] [PubMed]
- Catto, M.; Aliano, R.; Carotti, A.; Cellamare, S.; Palluotto, F.; Purgatorio, R.; De Stradis, A.; Campagna, F. Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1,3-diones and indol-2-aryldiazenylmethylene-3-ones as beta-amyloid aggregation inhibitors. Eur. J. Med. Chem. 2010, 45, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Campagna, F.; Catto, M.; Purgatorio, R.; Altomare, C.D.; Carotti, A.; De Stradis, A.; Palazzo, G. Synthesis and biophysical evaluation of arylhydrazono-1H-2-indolinones as β-amyloid aggregation inhibitors. Eur. J. Med. Chem. 2011, 46, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Catto, M.; Arnesano, F.; Palazzo, G.; De Stradis, A.; Calò, V.; Losacco, M.; Purgatorio, R.; Campagna, F. Investigation on the Influence of (Z)-3-(2-(3-Chlorophenyl)hydrazono)-5,6-dihydroxyindolin-2-one (PT2) on β-amyloid(1-40) Aggregation and Toxicity. Arch. Biochem. Biophys. 2014, 560, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; De Palma, A.; Giangregorio, N.; Miniero, D.V.; Pesce, P.; Nicolotti, O.; Campagna, F.; Altomare, C.D.; Catto, M. Mannich base approach to 5-methoxyisatin 3-(4-isopropylphenyl)hydrazone: A water-soluble prodrug for a multitarget inhibition of cholinesterases, beta-amyloid fibrillization and oligomer-induced cytotoxicity. Eur. J. Pharm. Sci. 2017, 109, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Saladino, R. A New Efficient and Mild Synthesis of 2-Oxindoles by One-Pot Wolff-Kishner Like Reduction of Isatin Derivatives. Synth. Commun. 1994, 24, 2835–2841. [Google Scholar] [CrossRef]
- Kondratieva, M.L.; Pepeleva, A.V.; Belskaia, N.P.; Koksharov, A.V.; Groundwater, P.V.; Robeyns, K.; Van Meervelt, L.; Dehaen, W.; Fan, Z.J.; Bakulev, V.A. A New Synthetic Method for the 2H-[1,2,3] Thiadiazolo[5,4-b]indoles. Tetrahedron 2007, 63, 3042–3048. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, S.K.; Singh, S.; Kumar, A.; Sharma, S. Synthesis and evaluation of novel indolylthiadiazinoazetidinones and indolylthiadiazinothiazolidinones as antimicrobial agents. Arch. Pharm. 2010, 343, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Muzalevskiy, V.M.; Balenkova, E.S.; Shastin, A.V.; Magerramov, A.M.; Shikhaliev, N.G.; Nenajdenko, V.G. New method for the preparation of 3-diazo-1,3-dihydroindol-2-ones. Russ. Chem. Bull. 2011, 60, 2343–2346. [Google Scholar] [CrossRef]
- Yoshino, T.; Shibata, K.; Wada, H.; Bando, Y.; Ishikawa, K.; Takezoe, H.; Mori, T. Organic field-effect transistors based on solution-processible dibenzotetrathiafulvalene derivatives. Chem. Lett. 2009, 38, 200–201. [Google Scholar] [CrossRef]
- Lackey, K.; Sternbach, D.D. Synthesis of substituted quinoline-4-carboxylic acids. Synthesis 1993, 10, 993–997. [Google Scholar] [CrossRef]
- Sandmeyer, T. Über Isonitrosoacetanilide und deren Kondensation zu Isatinen. Helv. Chim. Acta 1919, 2, 234–242. [Google Scholar] [CrossRef]
- Dahiya, R.; Narayan, S.; Bindal, V.; Kumar, V.; Handa, R.N.; Pujari, H.K. Heterocyclic Systems Containing Bridgehead Nitrogen Atom: Part LX—Synthesis of Thiazolo<2′,3′:3,4><1,2,4>triazino<5,6-b>indoles. Indian J. Chem. Sect. B 1987, 26B, 535–538. [Google Scholar]
- Gui, Q.; Dai, F.; Liu, J.; Chen, P.; Yang, Z.; Chen, X.; Tan, Z. Synthesis of N-alkyl isatins via oxidative cyclization of N-alkyl 2-bromo(chloro)acetanilides. Org. Biomol. Chem. 2014, 12, 3349–3353. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.J.; Fröhlich, R.; Oestreich, M. Stereochemical surprises in the Lewis acid-mediated allylation of isatins. J. Org. Chem. 2010, 75, 6720–6723. [Google Scholar] [CrossRef] [PubMed]
- Soto-Otero, R.; Mendez-Alvarez, E.; Sanchez-Iglesias, S.; Zubkov, F.I.; Voskressensky, L.G.; Varlamov, A.V.; de Candia, M.; Altomare, C.D. Inhibition of 6-hydroxydopamine-induced oxidative damage by 4,5-dihydro-3H-2-benzazepine N-oxides. Biochem. Pharmacol. 2008, 75, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Soto-Otero, R.; Mendez-Alvarez, E.; Sanchez-Iglesias, S.; Labandeira-García, J.L.; Rodríguez-Pallares, J.; Zubkov, F.I.; Zaytsev, V.P.; Voskressensky, L.G.; Varlamov, A.V.; de Candia, M.; et al. 2-Benzazepine nitrones protect dopaminergic neurons against 6-hydroxydopamine-induced oxidative toxicity. Arch. Pharm. 2012, 345, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.C.; Carr, P.-W.; Fréchet, J.M.; Smigol, V. Liquid chromatographic study of solute hydrogen bond basicity. Anal. Chem. 1994, 66, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.; Degennaro, L.; Carlucci, C.; de Candia, M.; Mastrorilli, P.; Roller, A.; Holzer, W.; Altomare, C.D.; Pace, V.; Luisi, R. A greener and efficient access to substituted four- and six-membered sulfur-bearing heterocycles. Org. Biomol. Chem. 2017, 15, 5000–5015. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.C.; Carr, P.-W. Extra-thermodynamic relationships in chromatography-study of the relationship between the slopes and intercepts of plots of log k’ vs. mobile phase composition in reversed-phase chromatography. J. Chromat. A 1993, 656, 521–535. [Google Scholar] [CrossRef]
- Kubinyi, H. Quantitative structure-activity relationships. 7. The bilinear model, a new model for nonlinear dependence of biological activity on hydrophobic character. J. Med. Chem. 1977, 20, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Hawkes, N. Pfizer abandons research into Alzheimer’s and Parkinson’s diseases. BMJ 2018, 360, k122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 4–11, 13–19, 21–24, 26, 28, 32–34, 36–39 are available from the authors. |





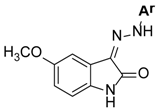 | ||
|---|---|---|
| Comp. | Ar | IC50 (µM) or (% Inhibition @ 100 µM) 1 |
| 1 | 4-(CH(CH3)2)C6H4 | 0.43 ± 0.04 |
| 4 | 4-CNC6H4 | (16 ± 3) |
| 5 | 4-CF3C6H4 | (57 ± 5) |
| 6 | 3,5-(CF3)2C6H3 | 9.9 ± 0.8 |
| 7 | Pyrid-2-yl | 90 ± 5 |
| 8 | 1-Naphthyl | 28 ± 2 |
| 9 | Quinolin-2-yl | (67 ± 4) |
| 10 | Quinolin-8-yl | (58 ± 4) |
| 11 | 7-Cl-quinolin-4-yl | (14 ± 2) |
 | |||
|---|---|---|---|
| Comp. | R | R1 | IC50 (µM) or (% Inhibition @ 100 µM) 1 |
| 13 | H | CH2Cl | 13 ± 3 |
| 14 | CH3 | CH3 | (43 ± 1) |
| 15 | H | C6H5 | 1.2 ± 0.2 |
| 16 | H | 3′-OHC6H4 | 16 ± 2 |
| 17 | H | 3′-OCH3C6H4 | 34 ± 6 |
| 18 | H | 3′-BrC6H4 | 40 ± 2 |
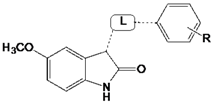 | |||
|---|---|---|---|
| Comp. | R | L | IC50 (µM) or (% inhibition @ 100 µM) 1 |
| 19 | 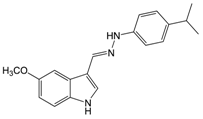 | 41 ± 3 | |
| 21 | 4-CH(CH3)2 | 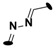 | 6.7 ± 0.2 |
| 22 | 3-Cl | 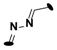 | 11 ± 2 |
| 23 | 3-Cl | 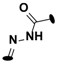 | (31 ± 3) |
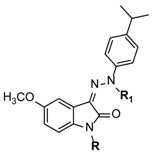 | |||
|---|---|---|---|
| Comp. | R | R1 | IC50 (µM) 1 |
| 24 | CH3 | CH3 | 11 ± 2 |
| 26 | CH3 | H | 0.81 ± 0.11 |
| 28 | Cyclopropyl | H | 0.80 ± 0.13 |
| 32 | Benzyl | H | 0.53 ± 0.10 |
| 33 | 2-Phenylethyl | H | 0.61 ± 0.03 |
| 34 | 3-Phenylpropyl | H | 0.83 ± 0.20 |
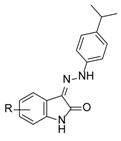 | ||
|---|---|---|
| Comp. | R | % Inhibition @ 100 µM 1 |
| 36 | 5-n-Butyl | 55 ± 1 |
| 37 | 7-Br | <5 |
| 38 | 5-SO3−Na+ | 35 ± 4 |
| 39 | 5,6-methylenedioxy | 70 ± 4 |
| Comp. | pIC50 | log k’w | CMR | MV |
|---|---|---|---|---|
| 1 | 6.40 | 5.44 | 8.288 | 2.304 |
| 6 | 5.00 | 6.29 | 8.475 | 2.657 |
| 7 | 4.05 | 2.34 | 7.369 | 1.942 |
| 8 | 4.55 | 5.15 | 9.113 | 2.413 |
| 13 | 4.89 | 3.95 | 8.149 | 2.019 |
| 15 | 5.92 | 5.10 | 9.738 | 2.445 |
| 16 | 4.80 | 4.58 | 9.824 | 2.417 |
| 17 | 4.47 | 5.29 | 10.320 | 2.661 |
| 18 | 4.40 | 5.67 | 10.494 | 2.570 |
| 19 | 4.39 | 6.42 | 9.331 | 2.681 |
| 21 | 5.17 | 4.82 | 9.331 | 2.681 |
| 22 | 4.96 | 4.21 | 8.445 | 2.309 |
| 24 | 4.96 | 4.23 | 9.935 | 2.946 |
| 26 | 6.09 | 5.96 | 9.402 | 2.733 |
| 28 | 6.10 | 6.36 | 10.088 | 2.778 |
| 32 | 6.28 | 7.44 | 11.931 | 3.421 |
| 33 | 6.21 | 7.24 | 12.392 | 3.581 |
| 34 | 6.08 | 7.87 | 12.853 | 3.741 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purgatorio, R.; De Candia, M.; De Palma, A.; De Santis, F.; Pisani, L.; Campagna, F.; Cellamare, S.; Altomare, C.D.; Catto, M. Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity. Molecules 2018, 23, 1544. https://doi.org/10.3390/molecules23071544
Purgatorio R, De Candia M, De Palma A, De Santis F, Pisani L, Campagna F, Cellamare S, Altomare CD, Catto M. Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity. Molecules. 2018; 23(7):1544. https://doi.org/10.3390/molecules23071544
Chicago/Turabian StylePurgatorio, Rosa, Modesto De Candia, Annalisa De Palma, Francesco De Santis, Leonardo Pisani, Francesco Campagna, Saverio Cellamare, Cosimo Damiano Altomare, and Marco Catto. 2018. "Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity" Molecules 23, no. 7: 1544. https://doi.org/10.3390/molecules23071544
APA StylePurgatorio, R., De Candia, M., De Palma, A., De Santis, F., Pisani, L., Campagna, F., Cellamare, S., Altomare, C. D., & Catto, M. (2018). Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity. Molecules, 23(7), 1544. https://doi.org/10.3390/molecules23071544









