Hydrophilic Interaction Liquid Chromatography-Electrospray Ionization Mass Spectrometry for Therapeutic Drug Monitoring of Metformin and Rosuvastatin in Human Plasma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. MS Detection Optimization
2.1.2. Chromatography
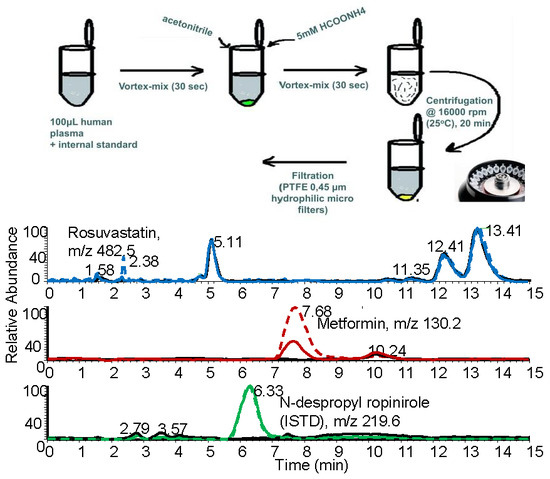
2.1.3. Optimization of the Preparation of the Biological Sample
2.2. Statistical Analysis of Data
2.2.1. Selectivity and Specificity
2.2.2. Linearity, Precision and Accuracy
2.2.3. Recovery and Matrix Effect
2.2.4. Stability
2.3. Application to the Analysis of Real Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Stock and Working Standard Solutions
3.4. Calibration Standards and Quality Control Samples
3.5. Sample Preparation Procedure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar]
- Bretnall, A.E.; Clarke, G.S. Metformin hydrochloride. Anal. Profil. Drug Sub. Excip. 1998, 25, 243–293. [Google Scholar]
- Zilinyi, R.; Czompa, A.; Czegledi, A.; Gajtko, A.; Pituk, D.; Lekli, I.; Tosaki, A. The Cardioprotective Effect of Metformin in Doxorubicin-Induced Cardiotoxicity: The Role of Autophagy. Molecules 2018, 23, 1184. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.Y.; Zhou, R.R.; Han, S.; Wang, T.S.; Wang, L.Q.; Xie, X.H. Statin therapy on glycemic control in type 2 diabetic patients: A network meta-analysis. J. Clin. Pharm. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, F. Comparative pharmacology of rosuvastatin. Atheroscl. Suppl. 2003, 4, 9–14. [Google Scholar] [CrossRef]
- Lee, D.; Roh, H.; Son, H.; Jang, S.B.; Lee, S.; Nam, S.Y.; Park, K. Pharmacokinetic interaction between rosuvastatin and metformin in healthy Korean male volunteers: A randomized, open-label, 3-period, crossover, multiple-dose study. Clin. Ther. 2014, 36, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Galviz, G.; Hwa, J.J. Ultra-performance hydrophilic interaction LC–MS/MS for the determination of metformin in mouse plasma. Bioanalysis 2009, 1, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, F.; Zhao, H.; Lin, Z.J.; Huang, Q.M.; Weng, N. Determination of metformin in rat plasma by HILIC-MS/MS combined with Tecan automation and direct injection. Biomed. Chromatogr. 2012, 26, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hander, G.M.; Makahleh, A.; Saad, B.; Saleh, M.I. Hollow fiber liquid phase microextraction with in situ derivatization for the determination of trace amounts of metformin hydrochloride (antidiabetic drug) in biological fluids. J. Chromatogr. B 2013, 941, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.; Christensen, M.M.H.; Brosen, K. Quantitation of metformin in human plasma and urine by hydrophilic interaction liquid chromatography and application to a pharmacokinetic study. Therap. Drug Monitor. 2014, 36, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Michel, D.; Gaunt, M.C.; Arnason, T.; El-Aneed, A. Development and validation of fast and simple flow injection analysis–tandem mass spectrometry (FIA-MS/MS) for the determination of metformin in dog serum. J. Pharm. Biomed. Anal. 2015, 107, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Bi, H.; Zhou, S.; Chen, X.; Huang, M. Simultaneous determination of metformin and gliclazide in human plasma by liquid chromatography-tandem mass spectrometry: Application to a bioequivalence study of two formulations in healthy volunteers. J. Mass Spectrom. 2005, 40, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Mistri, H.N.; Jangid, A.G.; Shrivastav, P.S. Liquid chromatography tandem mass spectrometry method for simultaneous determination of antidiabetic drugs metformin and glyburide in human plasma. J. Pharm. Biomed. Anal. 2007, 45, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Georgita, C.; Albu, F.; David, V.; Medvedovici, A. Simultaneous assay of metformin and glibenclamide in human plasma based on extraction-less sample preparation procedure and LC/(APCI)MS. J. Chromatogr. B 2007, 854, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pontarolo, R.; Gimenez, A.C.; Francisco, T.M.G.; Ribeiro, R.P.; Pontes, F.L.D.; Gasparetto, J.C. Simultaneous determination of metformin and vildagliptin in human plasma by a HILIC-MS/MS method. J. Chromatogr. B 2014, 965, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hander, G.M.; Makahleh, A.; Saad, B.; Saleh, M.I.; Cheng, K.W. Sequential hollow-fiber liquid phase microextraction for the determination of rosiglitazone and metformin hydrochloride (anti-diabetic drugs) in biological fluids. Talanta 2015, 131, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.I.; Mohamed, F.A.F.; Ahmed, S.; Mohamed, Y.A.S. An efficient hydrophilic interaction liquid chromatographic method for the simultaneous determination of metformin and pioglitazone using high-purity silica column. J. Chromatogr. B 2015, 997, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Vernikovskaya, D.I.; Fokina, V.M.; Nanovskaya, T.N.; Hankins, G.D.V.; Ahmed, M.S. Quantitative determination of metformin, glyburide and its metabolites in plasma and urine of pregnant patients by LC-MS/MS. Biomed. Chromatogr. 2015, 29, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Fachi, M.M.; Cerqueira, L.B.; Leonart, L.P.; Guimarães de Francisco, T.M.; Pontarolo, R. Simultaneous quantification of antidiabetic agents in human plasma by a UPLC QToF-MS method. PLoS ONE 2016, 11, e0167107. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Shah, J.V.; Sanyal, M.; Shrivastav, P.S. LC-MS/MS analysis of metformin, saxagliptin and 5-hydroxy saxagliptin in human plasma and its pharmacokinetic study with a fixed-dose formulation in healthy Indian subjects. Biomed. Chromatogr. 2017, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, M.L.; Moreira, F.L.; Ruela, A.L.M.; Santos, A.L.A.; Salgado, H.R.N.; Araújo, M.B. Analytical methods for the determination of rosuvastatin in pharmaceutical formulations and biological fluids: A critical review. Crit. Rev. Anal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.K.; Penman, A.D.; Smith, C.K.; Martin, P.D. Quantification of rosuvastatin in human plasma by automated solid phase extraction using tandem mass spectrometric detection. J. Chromatogr. B 2002, 772, 219–228. [Google Scholar] [CrossRef]
- Xu, D.H.; Ruan, Z.R.; Zhou, Q.; Yuan, H.; Jiang, B. Quantitative determination of rosuvastatin in human plasma by liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Oudhoff, K.A.; Sangster, T.; Thomas, E.; Wilson, I.D. Application of microbore HPLC in combination with tandem MS for the quantification of rosuvastatin in human plasma. J. Chromatogr. B 2006, 832, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhong, D.; Duan, X.; Chen, X. Liquid chromatography/negative ion electrospray tandem mass spectrometry method for the quantification of rosuvastatin in human plasma: Application to a pharmacokinetic study. J. Chromatogr. B 2007, 856, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Jiang, X.; Li, Y.; Wang, L.; Zhou, J.; Jiang, O.; Ye, L. Quantitative determination of rosuvastatin in human plasma by ion pair liquid-liquid extraction using liquid chromatography with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Ho, C.S.; Hu, M.; Tomlinson, B.; Wong, C.K. Development and validation of a sensitive method for simultaneous determination of rosuvastatin and N-desmethyl rosuvastatin in human plasma using liquid chromatography/negative electrosprayionization/tandem mass spectrometry. Biomed. Chromatogr. 2013, 17, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Macwan, J.S.; Ionita, I.A.; Akhlaghi, F. A simple assay for the simultaneous determination of rosuvastatin acid, rosuvastatin-5S-lactone, and N-desmethyl rosuvastatin in human plasma using liquid chromatography–tandem mass spectrometry (LC/MS/MS). Anal. Bioanal. Chem. 2012, 402, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Iqbal, Z.; Ahmad, L.; Nazir, S.; Watson, D.G.; Khuda, F.; Khan, A.; Khan, M.I.; Khan, A.; Nasir, F. Determination of rosuvastatin and its metabolite N-desmethyl rosuvastatin in human plasma by liquid chromatography-high resolution mass spectrometry: Method development, validation, and application to pharmacokinetic study. J. Liq. Chromatogr. Rel. Technol. 2015, 38, 863–873. [Google Scholar] [CrossRef]
- Vittal, S.; Shitut, N.R.; Kumar, T.R.; Vinu, M.C.A.; Mullangi, R.; Srinivas, N.R. Simultaneous quantitation of rosuvastatin and gemfibrozil in human plasma by high-performance liquid chromatography and its application to a pharmacokinetic study. Biomed. Chromatogr. 2006, 20, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Narapusetti, A.; Bethanabhatla, S.S.; Sockalingam, A.; Repaka, N.; Saritha, V. Simultaneous determination of rosuvastatin and amlodipine in human plasma using tandem mass spectrometry: Application to disposition kinetics. J. Adv. Res. 2015, 6, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Stopfer, P.; Giessmann, T.; Hohl, K.; Sharma, A.; Ishiguro, N.; Taub, M.E.; Jungnik, A.; Gansser, D.; Ebner, T.; Muller, F. Effects of metformin and furosemide on rosuvastatin pharmacokinetics in healthy volunteers: Implications for their use as probe drugs in a transporter cocktail. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.; Murthy, T.E.G.K.; Rao, M.V.B. Development, validation of liquid chromatography-tandem mass spectrometry method for simultaneous determination of rosuvastatin and metformin in human plasma and its application to a pharmacokinetic study. J. Adv. Pharm. Technol. Res. 2015, 6, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Machairas, G.; Panderi, I.; Geballa-Koukoula, A.; Rozou, S.; Antonopoulos, N.; Charitos, C.H.; Vonaparti, A. Development and validation of a hydrophilic interaction liquid chromatography method for the quantitation of impurities in fixed-dose combination tablets containing rosuvastatin and metformin. Talanta 2018, 183, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pedrali, A.; Tengattini, S.; Marrubini, G.; Bavaro, T.; Hemström, P.; Massolini, G.; Terreni, M.; Temporini, C. Characterization of Intact Neo-Glycoproteins by Hydrophilic Interaction Liquid Chromatography. Molecules 2014, 19, 9070–9088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsen, E.; Leknes, S.; Wilson, S.R.; Lundanes, E. Liquid chromatography-mass spectrometry platform for both small neurotransmitters and neuropeptides in blood, with automatic and robust solid phase extraction. Sci. Rep. 2015, 5, 9308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiriazopoulos, E.; Zaharaki, S.; Vonaparti, A.; Vourna, P.; Panteri-Petratou, E.; Gennimata, D.; Lombardo, K.; Panderi, I. Quantification of three beta-lactam antibiotics in breast milk and human plasma by hydrophilic interaction liquid chromatography/positive-ion electrospray ionization mass spectrometry. Drug Test. Anal. 2017, 9, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). Guideline on Bioanalytical Method Validation; EMEA/CHMP/EWP/192217/2009 Rev. 1; European Medicines Agency: London, UK, 2015. [Google Scholar]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the metformin and rosuvastatin and N-despropyl ropinirole are not available from the authors but it can be purchased from the manufacturers mentioned in the materials section. |






| Compound | Concentration Range, ng mL−1 | Regression Equations a | r b | Standard Deviation | Sr c | |
|---|---|---|---|---|---|---|
| Slope | Intercept | |||||
| Mean of three calibration curves over a period of one month | ||||||
| Metformin | 62.5–5000 | RMtf = 0.00715 × CMtf + 0.066 | ≥0.996 | 2.2 × 10−4 | 4.3 × 10−4 | ≤0.10 |
| Rosuvastatin | 2–100 | RRsv = 0.01775 × CRsv − 0.0129 | ≥0.998 | 5.2 × 10−4 | 7.2 × 10−3 | ≤0.062 |
| Compound | Concentration (ng mL−1) | ||
|---|---|---|---|
| Metformin | |||
| Added Concentration | 62.5 | 500 | 5000 |
| Run 1 (mean ± s.d.) | 63.9 ± 6.1 | 489 ± 29 | 5045 ± 352 |
| Run 2 (mean ± s.d.) | 64.3 ± 5.1 | 42.9 ± 8.3 | 4698 ± 143 |
| Run 3 (mean ± s.d.) | 60.1 ± 6.0 | 488 ± 39 | 4768 ± 232 |
| Overall mean | 62.7 | 497.9 | 4837 |
| Intra-day CV (%) a | 9.1 | 7.6 | 5.3 |
| Inter-day CV (%) a | 1.8 | 1.0 | 3.0 |
| Overall accuracy Er% b | 0.4 | −0.4 | −3.3 |
| Rosuvastatin | |||
| Added Concentration | 2 | 10 | 100 |
| Run 1 (mean ± s.d.) | 2.31 ± 0.21 | 9.41 ± 0.33 | 104.1 ± 6.6 |
| Run 2 (mean ± s.d.) | 2.12 ± 0.22 | 9.61 ± 0.89 | 96.5 ± 5.7 |
| Run 3 (mean ± s.d.) | 2.01 ± 0.21 | 10.81 ± 0.46 | 96.2 ± 9.1 |
| Overall mean | 2.1 | 9.9 | 98.9 |
| Intra-day CV (%) a | 8.5 | 6.2 | 7.3 |
| Inter-day CV (%) a | 4.4 | 7.1 | 3.0 |
| Overall accuracy Er% b | 7.0 | −1.0 | −1.1 |
| Compound | Concentration Levels (ng mL−1) | |
|---|---|---|
| Metformin | 1000 | 5000 |
| % Recovery (mean ± s.d.)n = 3 | 92.6 ± 2.5 | 93.9 ± 1.4 |
| % Matrix Factor (mean ± s.d.)n = 3 | 59.9 ± 1.0 | 63.7 ± 1.3 |
| Rosuvastatin | 20 | 100 |
| % Recovery (mean ± s.d.)n = 3 | 96.6 ± 2.1 | 95.8 ± 4.1 |
| % Matrix Factor (mean ± s.d.)n = 3 | 84.5 ± 7.1 | 81.3 ± 2.0 |
| N-despropyl ropinirole (ISTD) | 3800 | |
| % Recovery (mean ± s.d.)n = 3 | 91.7 ± 3.8 | |
| % Matrix Factor (mean ± s.d.)n = 3 | 61.8 ± 1.8 | |
| Compound | Calculated Concentration (ng mL−1) | |||||
|---|---|---|---|---|---|---|
| Metformin | 50 | 1250 | 2500 | |||
| Mean ± S.D.(n = 3) | %Er a | Mean ± S.D.(n = 3) | %Er a | Mean ± S.D.(n = 3) | %Er a | |
| Ambient temperature/4 h | 50.4 ± 1.9 | 0.9 | 1271.5 ± 8.2 | 1.7 | 2522 ± 138 | 0.01 |
| −20 °C/4 weeks | 49.7 ± 2.1 | −0.6 | 1260.1 ± 0.80 | 0.8 | 2412 ± 18 | −0.04 |
| −20 °C/4 Freeze-thaw cycles | 51.21 ± 0.81 | 2.4 | 1282.3 ± 2.6 | 2.6 | 2386 ± 24 | −0.05 |
| Rosuvastatin | 2.5 | 25 | 50 | |||
| Ambient temperature/4 h | 2.509 ± 0.0089 | 0.4 | 24.72 ± 0.11 | −1.1 | 49.4 ± 3.4 | −0.9 |
| −20 °C/4 weeks | 2.457 ± 0.045 | −1.7 | 24.53 ± 0.15 | −1.9 | 50.2 ± 3.2 | 0.4 |
| −20 °C/4 Freeze-thaw cycles | 2.435 ± 0.031 | −2.6 | 24.94 ± 0.42 | −0.2 | 49.5 ± 2.4 | −1.0 |
| Patient Sex/Age (years) | Time Post Dose (h) | Drug/Dose Peros (mg) | C (ng mL−1) Mean ± S.D.(n = 3) | Co-Administered Drugs |
|---|---|---|---|---|
| ♀/57 | 21/2 | Metformin/1000 × 1 × 30 days Rosuvastatin/10 × 1 × 30 days | 1956 ± 93 22.2 ± 0.4 | Acetylsalicylic acid 100 mg tb 1 × 1 |
| ♂/64 | 11 | Rosuvastatin/20 × 1 × 30 days | 16.2 ± 1.4 | Furosemide 40 mg tb 1 × 1, ramipril 2.5 mg tb 1 × 1, carvedilol 6.35 mg tb 1 × 1. |
| ♂/63 | 16 | Rosuvastatin/20 × 1 × 30 days | 9.21 ± 0.39 | Nadroparin 5700 anti-ha 1 × 1 subcutaneous, omeprazole caps 20 mg 1 × 1, metoprolol tb 25 mg 1 × 2, sulbactam 1 g & ampicillin i.v. 1 × 3 × 5 days, acetyl salicylic acid 100 mg E.C.tb 1 × 1, atalopram 20 mg 1 × 1. |
| ♀/80 | 21/2 | Metformin/1000 × 1 × 30 days | 2635 ± 67 | Acetylsalicylic acid 100 mg tb 1 × 1, insuline glarine 100 iu/mL 10IU ×1 night subcutaneous, Ramipril 5 mg tb 1 × 1, nadropanin 2850 Anti-xa 1 × 1, piracetam i.v. 1 × 2. |
| ♀/83 | 10 | Metformin/1000 × 2 × 30 days | 1850 ± 55 | Furosemide 20 mg tb 1 × 1, 1 × 1, insuline glarine 100 iu/mL 14IU ×1 night subcutaneous, pantoprazole 40 mg caps 1 × 1, T4-50 1 × 1, carvedilol 12.5 mg tb 1 × 2, apixaban 2.5 mg tb 1 × 2, Lutein/Zeaxanthine/Mesozeaxanthine caps 1 × 1, VITAMIN D3 2000 IU 1 × 1. |
| ♂/70 | 21/2 | Metformin/850 × 2 × 30 days | 1856 ± 84 | Ezetimibe/simvastatin 10/40 mg tb 1 × 1, gliclazide 30 mg tb 1 × 1, sertraline 50 mg tb 1 × 2, galantamine 8 mg caps 1 × 1, levodopa/carvidopa/entacapone 100/25/200 tb 1 × 3, clopidogrel 75 mg tb 1 × 1. |
| ♀/88 | 12 | Metformin/850 × 1 × 30 days | 1676 ± 60 | Atorvastatin tb 40 mg 1 × 1, furosemide 20 mg i.v. 2 × 3, bisoprostol 10 mg 1 × 1, insuline glarine 100 iu/mL 10IU ×1 night subcutaneous, thyrormone 100 mg 1 × 1, acetyl salicylic acid 100 mg E.C.tb 1 × 1, digoxin tb 0.25 mg ½ × 1. |
| ♀/88 | 16 | Metformin/850 × 1 × 30 days | 226 ± 12 | Atorvastatin tb 40 mg 1 × 1, furosemide 20 mg i.v. 2 × 3, insuline glarine 100 iu/mL 10IU ×1 night subcutaneous, thyrormone 100 mg 1 × 1, digoxin tb 0.25 mg ½ × 1, acetyl salicylic acid 100 mg E.C. tb 1 × 1. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonopoulos, N.; Machairas, G.; Migias, G.; Vonaparti, A.; Brakoulia, V.; Pistos, C.; Gennimata, D.; Panderi, I. Hydrophilic Interaction Liquid Chromatography-Electrospray Ionization Mass Spectrometry for Therapeutic Drug Monitoring of Metformin and Rosuvastatin in Human Plasma. Molecules 2018, 23, 1548. https://doi.org/10.3390/molecules23071548
Antonopoulos N, Machairas G, Migias G, Vonaparti A, Brakoulia V, Pistos C, Gennimata D, Panderi I. Hydrophilic Interaction Liquid Chromatography-Electrospray Ionization Mass Spectrometry for Therapeutic Drug Monitoring of Metformin and Rosuvastatin in Human Plasma. Molecules. 2018; 23(7):1548. https://doi.org/10.3390/molecules23071548
Chicago/Turabian StyleAntonopoulos, Nikolaos, Giorgos Machairas, George Migias, Ariadni Vonaparti, Vasiliki Brakoulia, Constantinos Pistos, Dimitra Gennimata, and Irene Panderi. 2018. "Hydrophilic Interaction Liquid Chromatography-Electrospray Ionization Mass Spectrometry for Therapeutic Drug Monitoring of Metformin and Rosuvastatin in Human Plasma" Molecules 23, no. 7: 1548. https://doi.org/10.3390/molecules23071548