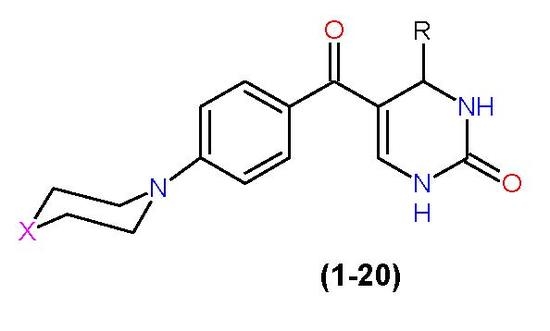
A One-Pot Biginelli Synthesis and Characterization of Novel Dihydropyrimidinone Derivatives Containing Piperazine/Morpholine Moiety
Abstract
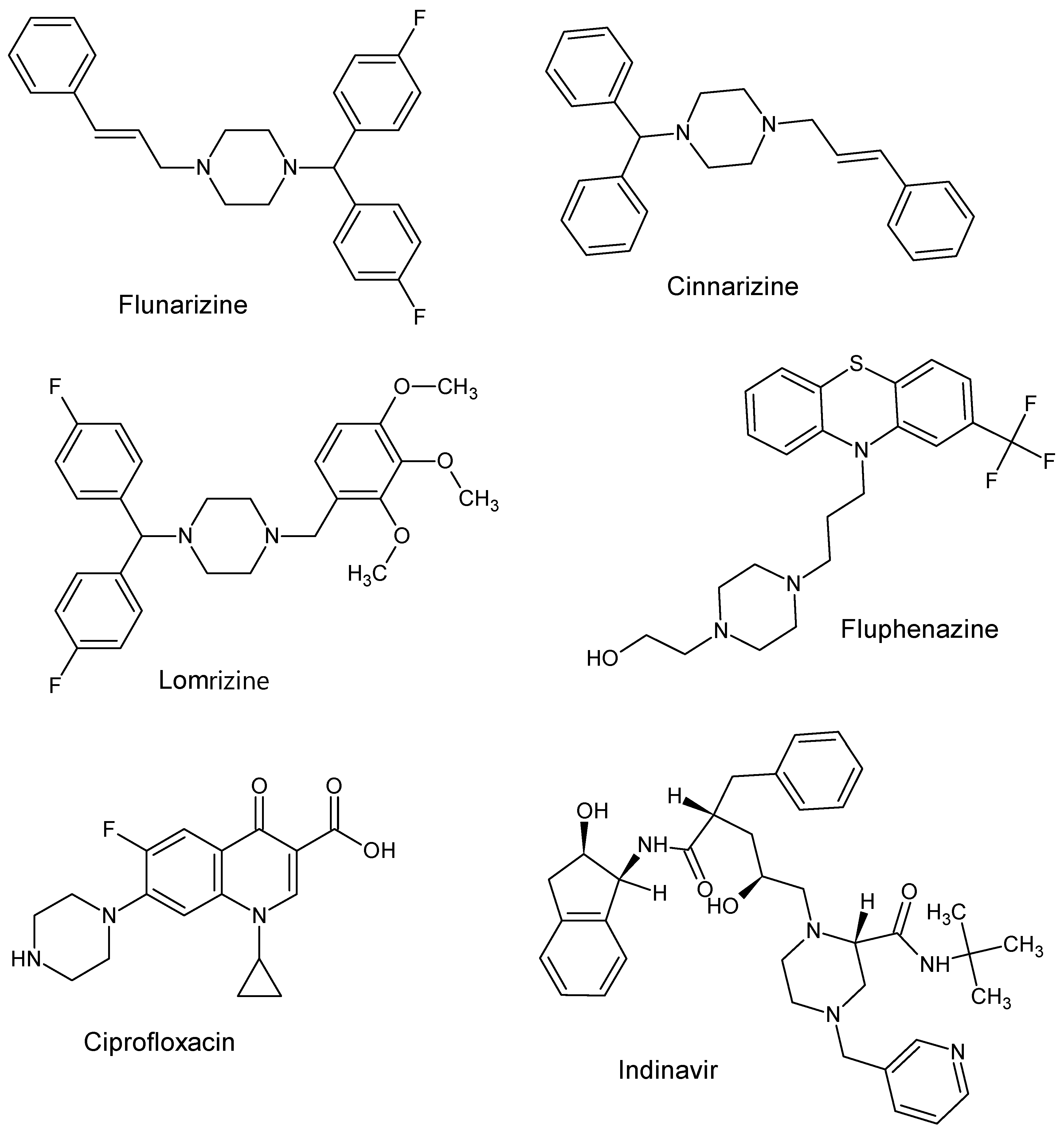
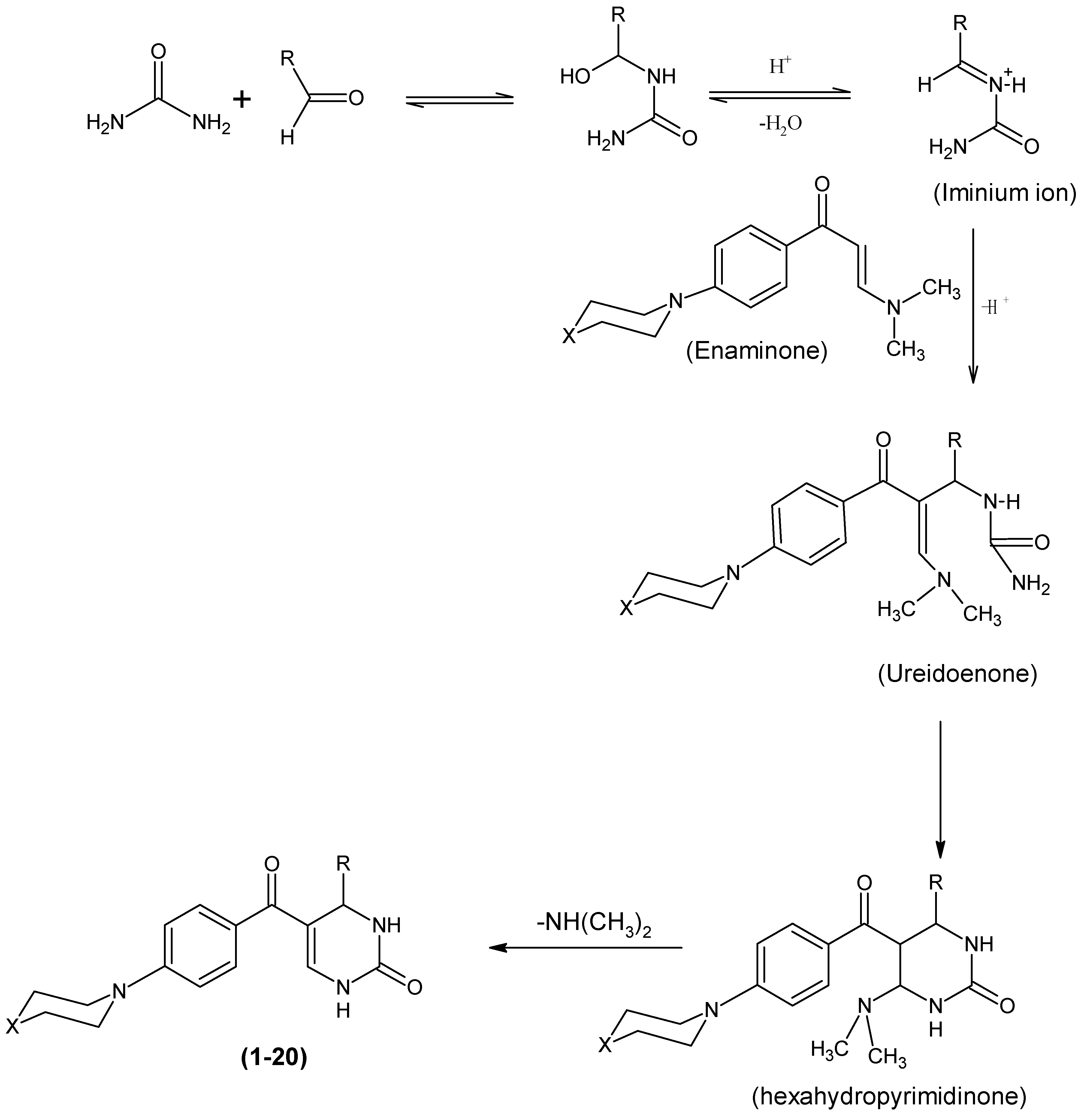
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. Chemistry
3.2. Synthesis of 3-(dimethylamino)-1-(4-(piperazin-1-yl) phenyl)prop-2-en-1-one (IIa)
3.3. Synthesis of 3-(dimethylamino)-1-(4-morpholinophenyl)prop-2-en-1-one (IIb)
3.4. General Synthesis of 4-(substituted phenyl)-5-[4-(piperazin/morpholin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (1–20)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Folkers, K.; Harwood, H.J.; Johnson, T.B. Researches on pyrimidines. cxxx. Synthesis of 2-keto-1,2,3,4-tetrahydropyrimidines. J. Am. Chem. Soc. 1932, 54, 3751–3758. [Google Scholar] [CrossRef]
- Atwal, K.S.; Ahmed, S.Z.; Bird, J.E.; Delaney, C.L.; Dickinson, K.E.; Ferrara, F.N.; Hedberg, A.; Miller, A.V.; Moreland, S.; O’Reilly, B.C.; et al. Dihydropyrimidine angiotensin II receptor antagonists. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1992, 35, 4751–4763. [Google Scholar] [CrossRef] [PubMed]
- Rovnyak, G.C.; Kimball, S.D.; Beyer, B.; Cucinotta, G.; DiMarco, J.D.; Gougoutas, J.; Hedberg, A.; Malley, M.; McCarthy, J.P.; Zhang, R.; et al. Calcium entry blockers and activators: Conformational and structural determinants of dihydropyrimidine calcium channel modulators. J. Med. Chem. 1995, 38, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Kaur, B.; Kumar, B. Synthesis and antihypertensive activity of some dihydropyrimidines. Ind. J. Chem. 2004, 43, 1553–1557. [Google Scholar] [CrossRef]
- Beena, K.P.; Suresh, R.; Rajasekaran, A.; Manna, P.K. DihydroPyrimidinones-A Versatile Scaffold with Diverse Biological Activity. J. Pharm. Sci. Res. 2016, 8, 741–746. [Google Scholar]
- Bhat, M.A.; Al-Dhfyan, A.; Al-Omar, M.A. Targeting Cancer Stem Cells with Novel 4-(4-2-substitutedphenyl)-5-(3,4,5-trimethoxy/3,4-dimethoxy)-3benzoyl-3,4-ihydropyrimidine-2(1H)-one/thiones. Molecules 2016, 21, 1746. [Google Scholar] [CrossRef] [PubMed]
- Atwal, K.S.; Swanson, B.N.; Unger, S.E.; Floyd, D.M.; Moreland, S.; Hedberg, A.; O’Reilly, B.C. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1991, 34, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. 100 Years of the Biginelli dihydropyridine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Bushra, B.A.; Zabiulla, M.S.V.; Mamatha, S.V.; Khanum, S.A. Piperazine and morpholine: Synthetic preview and pharmaceutical applications. J. Chem. Pharm. Res. 2015, 7, 281–301. [Google Scholar] [CrossRef]
- Patel, R.V.; Park, S.W. An evolving role of piperazine moieties in drug design and discovery. Mini-Rev. Med. Chem. 2013, 13, 1579–1601. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, M.L.; Ayala, D.; Benhamu, B.; Morcillo, M.J.; Viso, A. Arylpiperazine derivatives acting at 5-HT(1A) receptors. Curr. Med. Chem. 2002, 9, 443–469. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Leopoldo, M.; De Giorgio, P.; Berardi, F.; Perrone, R. Determination of 1-aryl-4-propylpiperazine pKa values: The substituent on aryl modulates basicity. Bioorg. Med. Chem. 2009, 17, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Haupt, A.; Pohlki, F.; Drescher, K.; Wicke, K.; Unger, L.; Relo, A.; Bespalov, A.; Vogg, B.; Backfisch, G.; Delzer, J.; et al. N-Phenyl-(piperazinyl or homopiperazinyl)-benzenesulfonamide or Benzenesulfonyl-phenyl-(piperazine or homopiperazine) Compounds Suitable for Treating Disorders that Respond to Modulation of the Serotonin 5-HT-6 Receptor. WO 2010125134 A1, 4 November 2010. [Google Scholar]
- Freire, T.R.V.; Mannochio, D.S.R.E. Compounds and Pharmaceutical Compositions for Treating Disorders Associated with the 5-HT1a and 5-HT2a Receptors. WO 2012037634 A1, 29 March 2012. [Google Scholar]
- Højer, A.M.; Drewes, P.G.; Kateb, J. New Compositions of 1-[2-(2,4-dimethyl-phenylsulfanyl)-phenyl]piperazine. WO 2011023194 A2, 3 March 2011. [Google Scholar]
- Ito, N.; Sasaki, H.; Tai, K.; Shinohara, T. Heterocyclic Compounds for Treating or Preventing Disorders Caused by Reduced Neurotransmission of Serotonin, Norephnephrine or Dopamine. WO 2012036253 A1, 22 March 2012. [Google Scholar]
- Bang-Andersen, B.; Mork, A.; Moore, N.; Stensbol, T. 1-[2-(2,4-Dimethylphenylsulfanyl)-phenyl]piperazine as a Compound with Combined Serotonin Reuptake, 5-HT3 and 5-HT1a Activity for the Treatment of Cognitive Impairment. U.S. Patent 20140248355 A1, 4 September 2014. [Google Scholar]
- Zhang, Y.; Jin, C.; Zhou, R. Preparation of Pyrimidinyl Piperazine Derivatives as Selective Serotonin Reuptake Inhibitors for the Treatment and Prevention of CNS Disorders. WO 2015014256 A1, 5 February 2015. [Google Scholar]
- Achanath, R.; Jose, J.; Rangaswamy, C.; Mandal, S.; Balaji, S.; KadavilpparampuIan, M.A.M.; Newington, I.M. Preparation of Piperazine Derivatives as Imaging Agents. WO 2013041682 A1, 28 March 2013. [Google Scholar]
- Hoenke, C.; Giovannini, R.; Lessel, U.; Rosenbrock, H.; Schmid, B. Piperazine Derivatives and the Use of Thereof as a Medicament. WO 2015055698 A1, 23 April 2015. [Google Scholar]
- Jin, L.; Yang, R.; Song, R.; Zhu, H.; Wu, N.; Yun, L.; Su, R.; Zhao, R. Preparation of Novel Piperazine Derivatives as Dopamine D3 Receptor Ligands. U.S. Patent 2014/0329831 A1, 6 November 2014. [Google Scholar]
- Ganesh, T.; Sun, A.; Smith, S.M.; Lambeth, J.D. Preparation of Piperazine Derivatives for Use as NADPH-Oxidase Inhibitors. WO 2012173952 A1, 20 December 2012. [Google Scholar]
- Yeung, K.S.; Farkas, M.E.; Kadow, J.F.; Meanwell, N.A.; Taylor, M.; Johnston, D.; Coulter, T.; Wright, J.J. Indole, Azaindole and Related Heterocyclic N-Substituted Piperazine Derivatives and Their Preparation and Use for the Treatment of HIV Infection. U.S. Patent 8039486 B2, 18 October 2011. [Google Scholar]
- Sofia, M.J.; Kakarla, R.; Liu, J.; Naduthambi, D.; Mosley, R.; Steuer, H.M. Preparation of Piperazine Derivatives and Their Uses to Treat Viral Infections, Including Hepatitis C. U.S. Patent 20120202794 A1, 9 August 2012. [Google Scholar]
- Carniato, D.; Briand, J.F.; Gutmann, M.; Busnel, O.; Bougeret, C.; Deprez, B.; Jaillardon, K. Preparation of Piperazine Derivatives Useful for the Treatment of Cancers, Especially Cancers Resistant to Chemotherapy. WO 2013098393 A1, 4 July 2013. [Google Scholar]
- Wang, S.; Zhou, H.; Chen, J.; Aguilar, A.; Meagher, J.L.; Sun, D.; Yang, C.; Liu, L.; Bai, L.; McEachem, D.; et al. Synthesis of Piperazine Derivatives as Bcl-2/Bcl-xL Inhibitors. U.S. Patent 20120189539 A1, 26 July 2012. [Google Scholar]
- Csonka, I.; Grolmusz, V.; Répási, J.; Szabadka, Z.; Szabo, A.; Kertesz, M. Piperazine Derivatives and Their Preparation and Use as Tuberculostatics. WO 2011089456 A1, 28 July 2011. [Google Scholar]
- Keliher, E.J.; Reiner, T.; Weissleder, R. Preparation of Substituted Piperazine Derivatives for Use in Targeting PARP-1 for Detection and Imaging of Cancer. WO 2012074840 A2, 7 June 2012. [Google Scholar]
- Kato, S.; Morie, T.; Hino, K.; Kon, T.; Naruto, S.; Yoshida, N.; Karasawa, T.; Matsumoto, J. Novel benzamides as selective and potent gastric prokinetic agents. 1. Synthesis and structure-activity relationships of N-[(2-Morpholinyl)alkyl]benzamides. J. Med. Chem. 1990, 33, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Chrysselis, M.C.; Rekka, E.A.; Kourounakis, P.N. Hypocholesterolemic and hypolipidemic activity of some novel morpholine derivatives with antioxidant Activity. J. Med. Chem. 2000, 43, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.J.; Mills, S.G.; MacCoss, M.; Dorn, C.P.; Finke, P.E.; Budhu, R.J.; Reamer, R.A.; Huskey, S.E.; Luffer-Atlas, D.; Dean, B.J.; et al. Phosphorylated morpholine acetal human neurokinin-1 receptor antagonists as water-soluble prodrugs. J. Med. Chem. 2000, 43, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Kuettel, S.; Zambon, A.; Kaiser, M.; Brun, R.; Scapozza, L.; Perozzo, R. Synthesis and evaluation of antiparasitic activities of new 4-[5-(4-Phenoxyphenyl)-2H-pyrazol-3-yl]morpholine derivatives. J. Med. Chem. 2007, 50, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Takaya, M.; Sato, M.; Terashima, K.; Tanizawa, H.; Maki, Y. A new Nonsteroidal analgesic-anti-inflammatory Agent. Synthesis and activity of 4-Ethoxy-2-methyl-5-morpholino-3(2H)-pyridazinone and related compounds. J. Med. Chem. 1979, 22, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Kuroda, T.; Uemori, S.; Moriguchi, A.; Ikeda, Y.; Hirayama, F.; Yokoyama, Y.; Iwao, E.; Yakushiji, T. Quinolone antimicrobial agents substituted with morpholines at the 7-Position, synthesis and structure-activity relationships. J. Med. Chem. 1993, 36, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Lukas, R.J.; Muresan, A.Z.; Damaj, M.I.; Blough, B.E.; Huang, X.; Navarro, H.A.; Mascarella, S.W.; Eaton, J.B.; Marxer-Miller, S.K.; Carroll, F.I. Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: Aids to smoking cessation. J. Med. Chem. 2010, 53, 4731–4748. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Ahmed, A.F.; Wen, Z.H.; Al-Omar, M.A.; Abdel-Aziz, H.A. Synthesis, anti-inflammatory and neuroprotective activity of pyrazole and pyrazolo[3,4-d]pyridazine bearing 3,4,5-trimethoxyphenyl. Med. Chem. Res. 2017, 26, 1557–1566. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Rashood, K.A.; Abdel-Aziz, H.A. Unexpected configuration in stereoslective synthesis of some novel (1Z)-1-(morpholin-1-yl)-N2-arylamidrazones. Lett. Org. Chem. 2012, 9, 487–492. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Omar, M.A.; Naglah, A.M. Synthesis and in vivo anti-ulcer evaluation of some novel piperidine linked dihydropyrimidinone derivatives. J. Enzym. Inhib. Med. Chem. 2018, 33, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhfyan, A.; Bhat, M.A. Method for Treating Cancer Using a Dihydropyrimidine Derivative. U.S. Patent 9,119,856 B1, 1 September 2015. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkins Trans. 1987, 12, S1–S19. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXTL-PC, version 5.1; Siemens Analytical Instruments, Inc.: Madison, WI, USA, 1997. [Google Scholar]
Sample Availability: Samples of the compounds (1–20) with 99% purity are available from authors. |


| Comp. | X | R |
|---|---|---|
| 1 | NH | C6H5– |
| 2 | NH | 2-NO2–C6H4– |
| 3 | NH | 4-NO2–C6H4– |
| 4 | NH | 3-NO2–C6H4– |
| 5 | NH | 4-Cl–C6H4– |
| 6 | NH | 2-OCH3–C6H4– |
| 7 | NH | 4-OH–C6H4– |
| 8 | NH | 3-OH–C6H4– |
| 9 | NH | 3-OCH3–C6H4– |
| 10 | NH | 4-OC2H5–C6H4– |
| 11 | O | C6H5– |
| 12 | O | 2-NO2–C6H4– |
| 13 | O | 4-NO2–C6H4– |
| 14 | O | 3-NO2–C6H4– |
| 15 | O | 4-Cl–C6H4– |
| 16 | O | 2-OCH3–C6H4– |
| 17 | O | 4-OH–C6H4– |
| 18 | O | 3-OH–C6H4– |
| 19 | O | 3-OCH3–C6H4– |
| 20 | O | 4-OC2H5–C6H4– |
| Crystal Data | |
|---|---|
| Chemical formula | C15H20N2O2 |
| Mr | 260.33 |
| Crystal system, space group | Triclinic, P-1 |
| Temperature (K) | 293 |
| a, b, c (Å) | 9.5268 (7), 10.2914 (8), 15.3140 (11) |
| α β γ (°) | 104.458 (3), 97.224 (3), 97.984 (3) |
| V (Å3) | 1419.51 (18) |
| Z | 4 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.08 |
| Crystal size (mm) | 0.61 × 0.31 × 0.28 |
| Data collection | |
| Diffractometer | Bruker APEX-II D8 venture diffractometer |
| Absorption correction | Multi-scan SADABS Bruker 2014 |
| Tmin, Tmax | 0.952, 0.977 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 27345, 5007, 2799 |
| Rint | 0.102 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.075, 0.239, 1.04 |
| No. of reflections | 5007 |
| No. of parameters | 348 |
| No. of restraints | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.34, −0.32 |
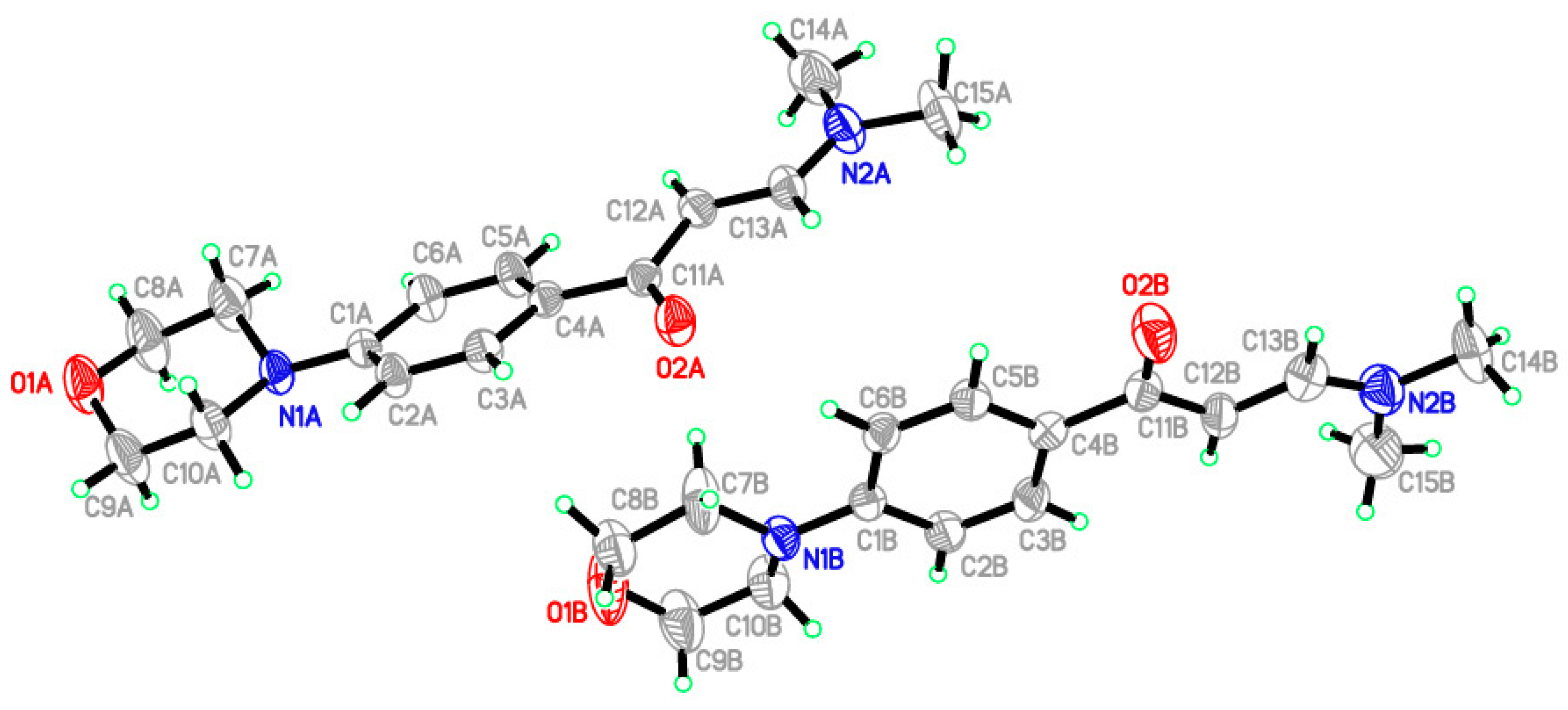
| O1A—C8A | 1.393 (5) | N2A—C13A | 1.326 (4) |
| O1A—C9A | 1.399 (5) | N2A—C14A | 1.429 (5) |
| O2A—C11A | 1.230 (4) | N2A—C15A | 1.443 (4) |
| O1B—C9B | 1.327 (6) | N1B—C1B | 1.402 (4) |
| O1B—C8B | 1.369 (5) | N1B—C7B | 1.406 (5) |
| O2B—C11B | 1.233 (5) | N1B—C10B | 1.427 (5) |
| N1A—C10A | 1.460 (4) | N2B—C13B | 1.332 (5) |
| N1A—C1A | 1.403 (4) | N2B—C14B | 1.440 (4) |
| N1A—C7A | 1.447 (5) | N2B—C15B | 1.453 (6) |
| C8A—O1A—C9A | 110.1 (3) | N1A—C7A—C8A | 111.8 (3) |
| C8B—O1B—C9B | 117.7 (3) | O1A—C8A—C7A | 113.1 (3) |
| C1A—N1A—C10A | 117.1 (2) | O1A—C9A—C10A | 112.6 (3) |
| C7A—N1A—C10A | 111.9 (3) | N1A—C10A—C9A | 111.4 (3) |
| C1A—N1A—C7A | 117.5 (2) | O2A—C11A—C4A | 118.5 (3) |
| C13A—N2A—C15A | 121.8 (3) | O2A—C11A—C12A | 123.1 (3) |
| C14A—N2A—C15A | 116.7 (3) | N2A—C13A—C12A | 127.7 (3) |
| C13A—N2A—C14A | 121.5 (3) | N1B—C1B—C2B | 121.2 (3) |
| C1B—N1B—C7B | 118.9 (3) | N1B—C1B—C6B | 122.0 (3) |
| C1B—N1B—C10B | 119.3 (3) | N1B—C7B—C8B | 115.9 (3) |
| C7B—N1B—C10B | 117.4 (3) | O1B—C8B—C7B | 116.9 (4) |
| C13B—N2B—C14B | 122.6 (3) | O1B—C9B—C10B | 119.2 (4) |
| C13B—N2B—C15B | 121.2 (3) | N1B—C10B—C9B | 115.8 (3) |
| C14B—N2B—C15B | 115.9 (3) | O2B—C11B—C4B | 119.0 (3) |
| N1A—C1A—C2A | 122.5 (3) | O2B—C11B—C12B | 121.6 (3) |
| N1A—C1A—C6A | 120.4 (3) | N2B—C13B—C12B | 128.1 (4) |
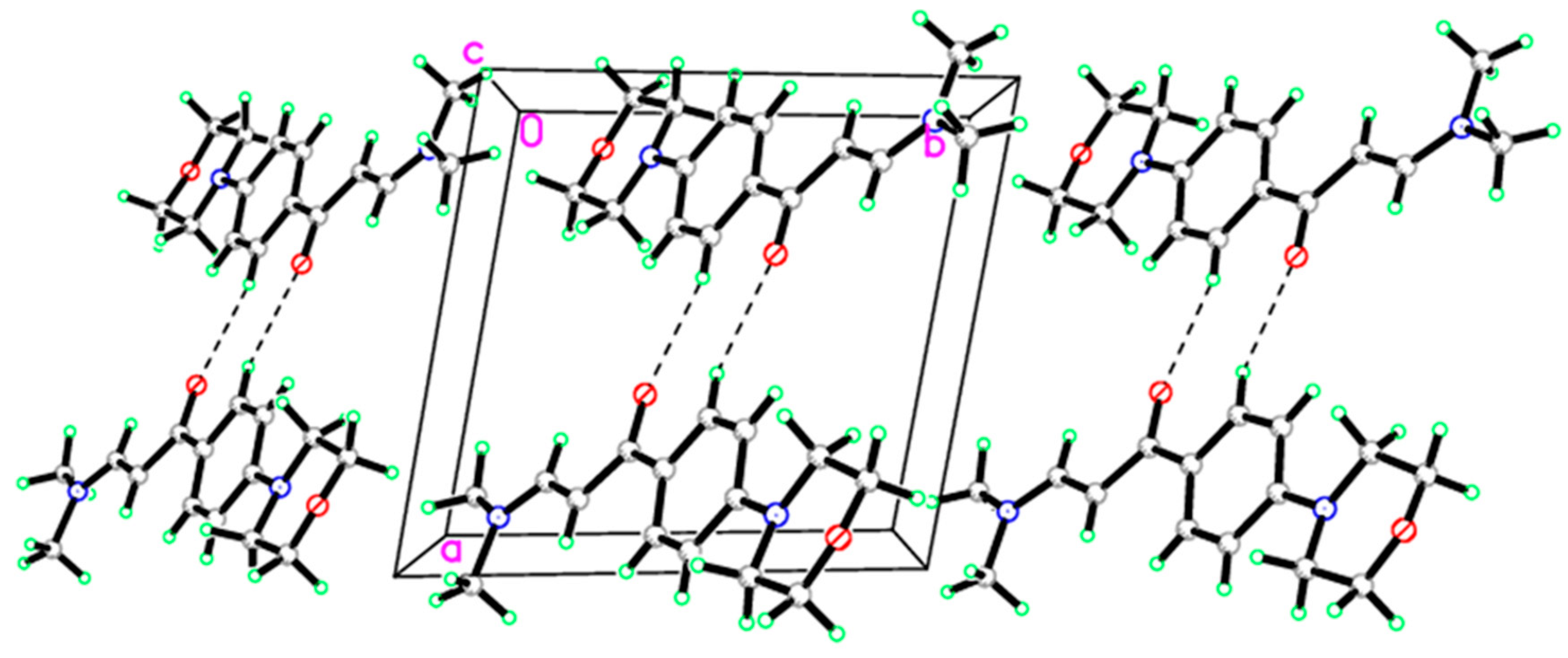
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| C5B—H5BA···O2Bi | 0.930 | 2.5100 | 3.391 (4) | 158.00 |
| C13A—H13A···O2Bi | 0.930 | 2.5900 | 3.451 (4) | 154.00 |
| C13B—H13B···O2Ai | 0.930 | 2.5800 | 3.418 (4) | 151.00 |
| C15A—H15A···O2Bi | 0.960 | 2.5100 | 3.375 (5) | 149.00 |
| Symmetry code: (i) –x + 1, −y + 1, −z. | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, M.A.; Al-Omar, M.A.; Ghabbour, H.A.; Naglah, A.M. A One-Pot Biginelli Synthesis and Characterization of Novel Dihydropyrimidinone Derivatives Containing Piperazine/Morpholine Moiety. Molecules 2018, 23, 1559. https://doi.org/10.3390/molecules23071559
Bhat MA, Al-Omar MA, Ghabbour HA, Naglah AM. A One-Pot Biginelli Synthesis and Characterization of Novel Dihydropyrimidinone Derivatives Containing Piperazine/Morpholine Moiety. Molecules. 2018; 23(7):1559. https://doi.org/10.3390/molecules23071559
Chicago/Turabian StyleBhat, Mashooq Ahmad, Mohamed A. Al-Omar, Hazem A. Ghabbour, and Ahmed M. Naglah. 2018. "A One-Pot Biginelli Synthesis and Characterization of Novel Dihydropyrimidinone Derivatives Containing Piperazine/Morpholine Moiety" Molecules 23, no. 7: 1559. https://doi.org/10.3390/molecules23071559
APA StyleBhat, M. A., Al-Omar, M. A., Ghabbour, H. A., & Naglah, A. M. (2018). A One-Pot Biginelli Synthesis and Characterization of Novel Dihydropyrimidinone Derivatives Containing Piperazine/Morpholine Moiety. Molecules, 23(7), 1559. https://doi.org/10.3390/molecules23071559