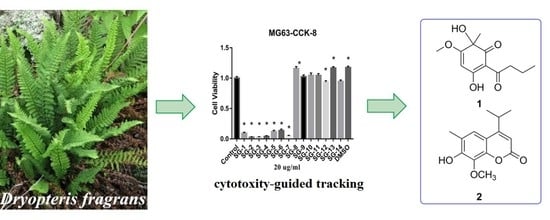
Cytotoxicity-Guided Isolation of Two New Phenolic Derivatives from Dryopteris fragrans (L.) Schott
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Isolated Compounds
2.2. In Vitro Cytotoxicity and Immunomodulatory Activity Detection
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Determination of Anti-Tumor Fraction of Dryopteris fragrans
3.4. Extraction and Isolation
3.5. Silicon Etherification Involved Isolation of 2 and 3
3.5.1. Silicon Etherification of the Mixture of Compounds 2 and 3
3.5.2. Desilication of Compound 2a
3.5.3. Desilication of Compound 3a
3.6. MTT and CCK-8 Assay
3.7. Immunoregulation Activity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kuang, H.; Zhang, Y.; Li, G.; Zeng, W.; Wang, H.; Song, Q. A new phenolic glycoside from the aerial parts of Dryopteris fragrans. Fitoterapia 2008, 79, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Patama, T.T.; Widen, C.J. Phloroglucinol derivatives from Dryopteris fuscoatra and D. hawaiiensis. Phytochemistry 1991, 30, 3305–3310. [Google Scholar] [CrossRef]
- Zhao, D.D.; Zhao, Q.S.; Liu, L.; Chen, Z.Q.; Zeng, W.M.; Lei, H.; Zhang, Y.L. Compounds from Dryopteris fragrans (L.) Schott with cytotoxic activity. Molecules 2014, 19, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zeng, W.M.; Li, G.Y.; Liu, G.Q.; Zhao, D.D.; Wang, J.; Zhang, Y.L. Characterization of a new sesquiterpene and antifungal activities of chemical constituents from Dryopteris fragrans (L.) Schott. Molecules 2013, 19, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Muranaka, T.; Mori, K.; Jin, Z.X.; Tokuda, H.; Nishino, H.; Yoshida, T. Ichthyotoxic phloroglucinol derivatives from Dryopteris fragrans and their anti-tumor promoting activity. Chem. Pharm. Bull. 2000, 31, 1190–1195. [Google Scholar] [CrossRef]
- Peng, B.; Bai, R.F.; Li, P.; Han, X.Y.; Wang, H.; Zhu, C.C.; Zeng, Z.P.; Chai, X.Y. Two new glycosides from Dryopteris fragrans with anti-inflammatory activities. J. Asian Nat. Prod. Res. 2016, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Wang, W.; Luo, M.; Li, C.Y.; Zu, Y.G.; Mu, P.S.; Fu, Y.J. Solvent-free microwave extraction of essential oil from Dryopteris fragrans and evaluation of antioxidant activity. Food Chem. 2012, 133, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, M.; Zu, Y.; Fu, Y.; Gu, C.; Wang, W.; Yao, L.; Efferth, T. Dryofragin, a phloroglucinol derivative, induces apoptosis in human breast cancer MCF-7 cells through ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2012, 199, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Jiang, C.; Hua, X.; Wang, T.; Chai, Y. Cell cycle arrest and apoptosis induced by aspidin PB through the p53/p21 and mitochondria-dependent pathways in human osteosarcoma cells. Anti Cancer Drugs 2015, 26, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wan, D.; Song, W. Dryofragin inhibits the migration and invasion of human osteosarcoma U2OS cells by suppressing MMP-2/9 and elevating TIMP-1/2 through PI3K/AKT and p38 MAPK signaling pathways. Anti Cancer Drugs 2016, 27, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, C.; Luo, M.; Wang, W.; Gu, C.; Zu, Y.; Li, J.; Efferth, T.; Fu, Y. Aspidin PB, a phloroglucinol derivative, induces apoptosis in human hepatocarcinoma HepG2 cells by modulating PI3K/Akt/GSK3β pathway. Chem. Biol. Interact. 2013, 201, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mu, F.; Li, C.; Wang, W.; Luo, M.; Fu, Y.; Zu, Y. Aspidin BB, a phloroglucinol derivative, induces cell cycle arrest and apoptosis in human ovarian HO-8910 cells. Chem. Biol. Interact. 2013, 204, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.C.; Zhao, D.D.; Liu, Z.D.; Jiang, S.; Zhang, Y.L. A new human cancer cell proliferation inhibition sesquiterpene, dryofraterpene A, from medicinal plant Dryopteris fragrans (L.) Schott. Molecules 2017, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Popovich, D.G.; Hu, C.; Kitts, D.D. Evaluation of viability assays for anthocyanins in cultured cells. Phytochem. Anal. 2008, 19, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Gong, Y.; Zhu, Y.; Li, A.; Dong, N.; Piao, Y.; Yuan, Y. The biological activity of H. pylori SlyD in vitro. Helicobacter 2013, 18, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Lee, D.Y.; Lee, J.W.; Lee, D.G.; Lee, Y.H.; Kim, S.Y.; Kim, S.H. Acyclic diterpenoids from the leaves of Capsicum annuum. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 128–132. [Google Scholar] [CrossRef]
- Amzad, H.M. Synthesis of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone. Bangladesh J. Sci. Ind. Res. 2001, 36, 50–54. [Google Scholar]
- Ayräs, P.; Lötjönen, S.; Widén, C.J. NMR spectroscopy of naturally occurring phloroglucinol derivatives. Planta Med. 1981, 42, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Echeverri, T.; Rivero-Cruz, J.F.; Su, B.N.; Chai, H.B.; Cordell, G.A.; Pezzuto, J.M.; Swanson, S.M.; Soejarto, D.D.; Kinghorn, A.D. Constituents of the leaves and twigs of Calyptranthes pallens collected from an experimental plot in Southern Florida. J. Nat. Prod. 2005, 68, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nisa, K.; Rakainsa, S.K.; Lallo, S.; Morita, H. New phloroglucinol derivatives from Indonesian Baeckea frutescens. Tetrahedron 2017, 73, 1177–1181. [Google Scholar] [CrossRef]
- Yang, L.; Guo, H.; Li, Y.; Meng, X.; Yan, L.; Dan, Z.; Wu, S.; Zhou, H.; Peng, L.; Xie, Q.; et al. Oleoylethanolamide exerts anti-inflammatory effects on LPS-induced THP-1 cells by enhancing PPARα signaling and inhibiting the NF-κB and ERK1/2/AP-1/STAT3 pathways. Sci. Rep. 2016, 6, 34611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |





| No. | δH | δC | No. | δH | δC |
|---|---|---|---|---|---|
| 1 | 196.32 (C) | 8a | 2.99 (1H, m) | 41.04 (CH2) | |
| 2 | 75.37 (C) | 8b | 2.92 (1H, m) | ||
| 3 | 176.04 (C) | 9 | 1.69 (2H, m) | 18.71 (CH2) | |
| 4 | 5.37 (1H, s) | 94.49 (CH) | 10 | 1.01 (3H, t, J = 7.4) | 14.07 (CH3) |
| 5 | 189.34 (C) | 11 | 1.54 (3H, s) | 30.20 (CH3) | |
| 6 | 104.49 (C) | 12 | 3.91 (3H, s) | 57.35 (CH3) | |
| 7 | 203.65 (C) |
| No. | 2 | 3 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | ||||
| 2 | 161.5 (C) | 161.1 (C) | ||
| 3 | 6.16 (1H, s) | 107.8 (CH) | 6.22 (1H, s) | 109.2 (CH) |
| 4 | 163.0 (C) | 163.0 (C) | ||
| 5 | 7.17 (1H, s) | 120.0 (CH) | 7.01 (1H, s) | 115.5 (CH) |
| 6 | 121.1 (C) | 127.5 (C) | ||
| 7 | 150.0 (C) | 147.8 (C) | ||
| 8 | 133.6 (C) | 136.7 (C) | ||
| 9 | 112.2 (C) | 114.5 (C) | ||
| 10 | 145.5 (C) | 141.3 (C) | ||
| 11 | 3.25 (1H, m) | 28.7 (CH) | 3.25 (1H, m) | 28.7 (CH) |
| 12 | 1.30 (3H, d, J = 6.8) | 22.1 (CH3) | 1.31 (3H, d, J = 6.8) | 21.9 (CH3) |
| 13 | 1.30 (3H, d, J = 6.8) | 22.1 (CH3) | 1.31 (3H, d, J = 6.8) | 21.9 (CH3) |
| 14 | 2.31 (3H, s) | 15.9 (CH3) | 2.32 (3H, s) | 16.3 (CH3) |
| 15 | 4.08 (3H, s) | 61.9 (OCH3) | 3.95 (3H, s) | 60.5 (OCH3) |
| Compound | HepG2 | A549 | HeLa | U251 | HOS | MG63 | U2OS | MB231 | SKBR-3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 45.86 ± 1.64 | 25.37 ± 2.62 | 46.13 ± 1.90 | - | - | - | - | - |
| 2 | 45.52 ± 3.21 | 47.70 ± 2.43 | 15.12 ± 4.01 | 46.14 ± 2.40 | - | - | - | 25.59 ± 2.30 | - |
| 3 | 48.39 ± 2.15 | 38.01 ± 3.56 | - | - | - | - | - | 38.09 ± 2.40 | - |
| 4 | 38.13 ± 1.03 | 37.41 ± 1.24 | 1.24 ± 0.08 | - | - | - | 47.56 ± 2.23 | 41.95 ± 2.35 | - |
| 5 | - | 49.74 ± 3.35 | - | - | - | - | - | - | - |
| 6 | - | 47.42 ± 2.25 | - | - | - | - | - | - | |
| 7 | - | 40.03 ± 0.98 | - | - | - | - | - | - | |
| 8 | 18.02 ± 0.89 | - | 17.76 ± 3.43 | 41.21 ± 1.35 | 43.67 ± 2.52 | 22.76 ± 2.65 | 36.36 ± 1.32 | 39.61 ± 1.50 | 33.40 ± 1.50 |
| Taxol a | 5.32 ± 0.12 | 3.46 ± 0.23 | 0.17 ± 0.02 | 5.02 ± 0.21 | 3.71 ± 0.33 | 5.86 ± 0.24 | 1.01 ± 0.03 | 6.23 ± 0.36 | 3.12 ± 0.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, L.; Duan, D.-H.; Zhang, Y.-H.; Huang, S.-X.; Chang, Y. Cytotoxicity-Guided Isolation of Two New Phenolic Derivatives from Dryopteris fragrans (L.) Schott. Molecules 2018, 23, 1652. https://doi.org/10.3390/molecules23071652
Zhang T, Wang L, Duan D-H, Zhang Y-H, Huang S-X, Chang Y. Cytotoxicity-Guided Isolation of Two New Phenolic Derivatives from Dryopteris fragrans (L.) Schott. Molecules. 2018; 23(7):1652. https://doi.org/10.3390/molecules23071652
Chicago/Turabian StyleZhang, Tong, Li Wang, De-Hua Duan, Yi-Hao Zhang, Sheng-Xiong Huang, and Ying Chang. 2018. "Cytotoxicity-Guided Isolation of Two New Phenolic Derivatives from Dryopteris fragrans (L.) Schott" Molecules 23, no. 7: 1652. https://doi.org/10.3390/molecules23071652