Impact of Alkaline H2O2 Pretreatment on Methane Generation Potential of Greenhouse Crop Waste under Anaerobic Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
2.2. Effect of Alkaline H2O2 Pretreatment
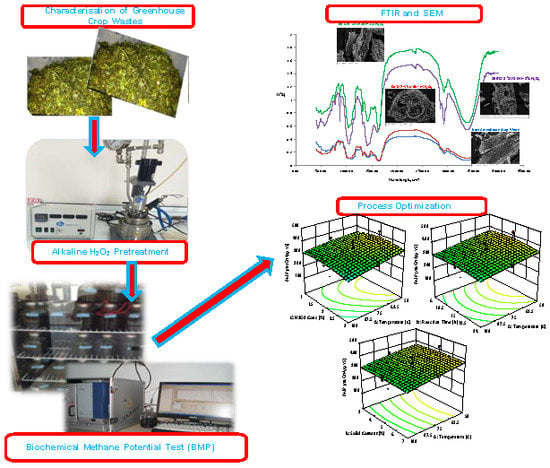
2.3. Alkaline H2O2 Pretreatment Process Optimization
2.4. Chemical Structure and Morphological Changes of Biomass
3. Materials and Methods
3.1. Experimental Rationale
3.2. Alkaline H2O2 Pretreatment Experiments
3.3. Methane Generation Potential Experiment
3.4. Optimization of the Alkaline H2O2 Pretreatment Process
3.5. Fourier-Transform Infrared (FTIR) Spectroscopy and Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herzog, A.V.; Lipman, T.E.; Kammen, D.M. Renewable Energy Sources. Encyclopedia of Life Support Systems (EOLSS) Forerunner: Paris, France. Available online: https://pdfs.semanticscholar.org/57ad/8c86294ae7af352177d11c28e6dad12db7de.pdf (accessed on 24 May 2018).
- Tüzel, Y.; Öztekin, G.B. Protected cultivation in Turkey. Chron. Horticult. 2015, 55, 21–26. [Google Scholar]
- Kacıra, M.; Sase, S.; Kacıra, O.; Okushima, L.; Ishii, M.; Kowata, H.; Moriyama, H. Status of greeenhouse production in Turkey: Focusing on vegetable and floriculture production. J. Agric. Meteorol. 2004, 60, 115–122. [Google Scholar] [CrossRef]
- Perendeci, N.A. Valorization of Agricultural Residues from Greenhouses and Sewage Sludge into Biogas by Anaerobic Digestion Combined with Pre-Treatments; TÜBİTAK Project Report; TÜBİTAK: Ankara, Turkey, 2010.
- Us, E.; Perendeci, A. Improvement of methane production from greenhouse residues: Optimization of thermal and H2SO4 pretreatment process by experimental design. Chem. Eng. J. 2012, 181, 120–131. [Google Scholar] [CrossRef]
- Mohareb, E.A.; MacLean, H.L.; Kennedy, C.A. Greenhouse gas emissions from waste management- Assesment of quantification methods. J. Air Waste Manag. Asooc. 2011, 61, 480–493. [Google Scholar] [CrossRef]
- Conde-Mejiaa, C.; Jiménez-Gutiérreza, A.; Halwagi, M.E. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Galbe, M.; Zacchi, G. Pretreatment of lignocellulosic materials for efficient bioethanol production. Biofuels 2007, 108, 41–65. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Eng. Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Sambusiti, C. Physical, Chemical and Biological Pretreatments to Enhance Biogas Production from Lignocellulosic Substrates. Ph.D. Thesis, Politecnico dı Milano, Milano, Italy, 2013. Available online: https://www.politesi.polimi.it/bitstream/10589/74843/3/2013_03_PhD_Sambusiti.pdf (accessed on 24 May 2018).
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Berlin, A.; Gilkes, N.; Kilburn, D.; Bura, R.; Robinson, J.; Markov, A.; Skomarovsky, A.; Gusakov, A.; Okunev, O.; et al. Enzymatic hydrolysis of steam-exploded and ethanol organasolv pretreated Douglas-Firby novel and commercial fungal cellulases. Appl. Biochem. Biotechnol. 2005, 121, 219–230. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J.J. Dilute acid pretreatment of rye straw and Bermuda grass for ethanol production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, R.; Cao, W.; Yin, R.; Mei, Y.; Zhang, L. Impacts of alkaline hydrogen peroxide pretreatment on chemical composition and biochemical methane potential of agricultural crop stalks. Energy Fuels 2015, 29, 4966–4975. [Google Scholar] [CrossRef]
- Qing, Q.; Zhou, L.; Huang, M.; Guo, Q.; He, Y.; Wang, L.; Zhang, Y. Improving enzymatic saccharification of bamboo shoot shell by alkaline salt pretreatment with H2O2. Bioresour. Technol. 2016, 201, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.M.; Sun, R.C.; Salisbury, D.; Fowler, P.; Tomkinson, J. Comparative study of hemicelluloses from wheat straw by alkali and hydrogen peroxide extractions. Polym. Degrad. Stab. 1999, 66, 423–432. [Google Scholar] [CrossRef]
- Michalska, K.; Ledakowicz, S. Alkaline hydrogen peroxide pretreatment of energy crops for biogas production. Chem. Pap. 2013, 68, 913–922. [Google Scholar] [CrossRef]
- Banerjee, G.; Car, J.; Scott-Craig, J.S.; Hodge, D.B.; Walton, J.B. Alkaline peroxide pretreatment of corn stover: Effects of biomass, peroxide, and enzyme loading and composition on yields of glucose and xylose. Biotechnol. Biofuels 2011, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, A.O.; Hymore, F.K.; Mudliar, S.N.; Deshmukh, S.C.; Satpute, D.B.; Omoleye, J.A.; Pandey, R.A. Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel 2011, 106, 187–194. [Google Scholar] [CrossRef]
- Vasco, C.A.; Zhang, X. Alkaline hydrogen peroxide pretreatment of softwood: Hemicellulose degradation pathways. Bioresour. Technol. 2013, 150, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.A.C.; Junior, J.E.M.; Gonçalves, L.R.B.; Rocha, M.V.P. Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: Study of parameters. Bioresour. Technol. 2013, 139, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, S.C.; Andrade, R.R.; Filho, R.M.; Costa, A.C. Alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis and fermentation of sugarcane bagasse to ethanol. Fuel 2014, 136, 349–357. [Google Scholar] [CrossRef]
- Su, Y.; Du, R.; Gu, H.; Ca, M.; Wu, Q.; Su, R.; Qi, W.; He, Z. Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: Characterization of its major components. Food Bioprod. Process 2015, 94, 322–330. [Google Scholar] [CrossRef]
- Zhang, H.; Ning, Z.; Khalid, H.; Zhang, R.; Liu, G.; Chen, C. Enhancement of methane production from cotton stalk using different pretreatment techniques. Sci. Rep. 2018, 8, 3463. [Google Scholar] [CrossRef] [PubMed]
- Orhon, D. Evolution of the activated sludge process: The first fifty years. J. Chem. Technol. Biotecnol. 2015, 90, 608–640. [Google Scholar] [CrossRef]
- Henze, M. Characterization of wastewater for modelling of activated sludge process. Water Sci. Technol. 1992, 25, 1–15. [Google Scholar] [CrossRef]
- Başaran, S.T.; Aysel, M.; Kurt, H.; Ergal, I.; Kumru, M.; Akarsubaşı, A.; Sözen, S.; Orhon, D. Removal of readily biodegradable substrate in super gas membrane reactor. J. Membr. Sci. 2012, 423, 477–486. [Google Scholar] [CrossRef]
- Sözen, S.; Çokgör, E.U.; Insel, G.; Taş, D.O.; Dülkadiroğlu, H.; Karaca, C.; Filibeli, A.; Meriç, S.; Orhon, D. Scientific basis of dissolved organic carbon limitation for landfilling of municipal treatment sludge—Is it attainable and justifiable? Waste Manag. 2014, 34, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Karaca, C.; Sözen, S.; Orhon, D.; Okutan, H. High temperature pyrolysis of sewage sludge as a sustainable process for energy recovery. Waste Manag. 2018, 78, 217–228. [Google Scholar] [CrossRef]
- Svardal, K.; Kroiss, H. Energy requirements for waste water treatment. Water Sci Technol. 2011, 64, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Rezende, C.A.; de Lima, M.A.; Maziero, P.; deAzevedo, E.R.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsianitis, D.; Mitani, C.; Giagli, K.; Tsalagkas, D.; Halász, K.; Kolonics, O.; Gallis, C.; Csóka, L. Properties of ultrasound extracted bicomponent lignocellulose thin films. Ultrason. Sonochem. 2015, 23, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Gabhane, J.; William, S.P.M.P.; Vaidya, A.N.; Das, S.; Wate, S.R. Solar assisted alkali pretreatment of garden biomass: Effects on lignocellulose degradation, enzymatic hydrolysis, crystallinity and ultra-structural changes in lignocellulose. Waste Manag. 2015, 40, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Song, W.; Ding, L.; Xie, B.; Zhou, J.; Cen, K. Characterization of water hyacinth with microwave-heated alkali pretreatment for enhanced enzymatic digestibility and hydrogen/methane fermentation. Bioresour. Technol. 2015, 182, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Van Soest, P.J. Use of detergent in the analysis of fibrous feeds. A rapid method for the determination of fibre and lignin. J. Assoc. Off. Anal. Chem. 1963, 46, 829–835. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and and Lignin in Biomass, 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 24 May 2015).
- Lowry, O.H.; Rosebrough, N.J.; Fau, A.L.; Randall, R.J. Protein measurement with the Folin reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Bridoux, G.; Dhulster, P.; Manem, J. Grease analysis on municipal waste water treatment. Plants Tech. Sci. Methods 1994, 5, 257–262. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Carrere, H.; Sialve, B.; Bernet, N. Improving pig manure conversioninto biogas by thermal and thermo-chemical pretreatments. Bioresour. Technol. 2009, 100, 3690–3694. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| Parameter | Result |
|---|---|
| Total solid, TS (g/kg) | 136.53 |
| Volatile solid, VS (g/kg) | 93.9 |
| Total Kjeldahl nitrogen, TKN (mg/gVS) | 6.75 |
| Protein (mg/gVS) | 60 |
| Chemical oxygen demand, COD (mg/gVS) | 1494.1 |
| Soluble chemical oxygen demand, sCOD (mg/gVS) | 60.88 |
| Soluble reducing sugar, sRedSugar (mg/gVS) | 7.59 |
| Extractable material and lipids * (%) | 0.14 |
| Van Soest fractionation | |
| Soluble matter (%) | 76.58 |
| Hemicellulose (%) | 3.89 |
| Cellulose (%) | 19.49 |
| Lignin (%) | 0.03 |
| Total lignin on an extractive free bases (%) | 19.39 |
| Acid-insoluble (%) | 17.25 |
| Acid-soluble (%) | 2.11 |
| Elemental Analysis | |
| C (%) | 29.23 |
| H (%) | 4.89 |
| N (%) | 2.96 |
| S (%) | 1.1 |
| sCOD Model | |||
| Quadratic model | |||
| Prob > F | <0.0001 Significant | Adj-R2 | 0.9562 |
| R2 | 0.9682 | Pred-R2 | 0.9338 |
| Adeq Precision | 35.6593 | C.V% | 8.85 |
| sCOD = +1045.11218 − 24.76191 × Reaction temp. − 44.99164 × Solid content + 88.00049 × H2O2 concent. − 3.98184 × Reaction time − 0.64327 × Reaction temp. × Solid content + 1.48441 × Reaction temp. × H2O2 concent. + 0.022507 × Reaction temp. × Reaction time − 27.45672 × Solid content × H2O2 concent. − 0.000607639 × Solid content × Reaction time − 0.62899 × H2O2 concent. × Reaction time + 0.19592 × Reaction Temp.2 + 11.76235 × Solid content2 + 6.39440 × H2O2 concent.2 + 0.21604 × Reaction time2. | |||
| sRedSugar Model | |||
| Quadratic model | |||
| Prob > F | <0.0001 Significant | Adj-R2 | 0.6966 |
| R2 | 0.7740 | Pred-R2 | 0.5519 |
| Adeq Precision | 11.705 | C.V% | 41.85 |
| sRedSugar = +844.41473 − 18.34946 × Reaction temp. + 16.89274 × Solid content − 136.48065 × H2O2 concent. – 13.05242 × Reaction time − 0.17577 × Reaction temp. × Solid content + 1.01831 × Reaction temp. × H2O2 concent. + 0.063115 × Reaction temp. × Reaction time − 2.93797 × Solid content × H2O2 concent. + 0.27415 × Solid content × Reaction time + 0.12308 × Reaction temp.2 − 1.32058 × Solid content2 + 18.98017 × H2O2 concent.2 + 0.29090 × Reaction time2 | |||
| Total Lignin on an Extractives Free Bases Model | |||
| Quadratic model | |||
| Prob > F | <0.0001 Significant | Adj-R2 | 0.7762 |
| R2 | 0.8376 | Pred-R2 | 0.6727 |
| Adeq Precision | 14.903 | C.V% | 14.18 |
| 1/(Lignin) = +0.0736566 + 5.8380149 × 10−5 × Reaction temp. − 0.0284772 × Solid content − 7.8491088 × 10−3 × H2O2 concent. − 4.5014496 × 10−4 × Reaction time + 3.4923132 × 10−5 × Reaction temp. × Solid content + 2.3900179 × 10−4 × Reaction temp. × H2O2 concent. − 2.2097257 × 10−7 × Reaction temp. × Reaction time + 8.6433922 × 10−4 × Solid content × H2O2 concent. − 4.3403413 × 10−5 × Solid content × Reaction time − 3.95154005 × 10−5 × H2O2 concent. × Reaction time − 6.01390484 × 10−6 × Reaction temp.2 + 2.664218431 × 10−3 × Solid content2 − 1.7142995 × 10−3 × H2O2 concent.2 + 2.74301920 × 10−5 × Reaction time2 | |||
| BMP Model | |||
| Quadratic model | |||
| Prob > F | <0.0001 Significant | Adj-R2 | 0.4112 |
| R2 | 0.5728 | Pred-R2 | 0.1190 |
| Adeq Precision | 7.23 | C.V% | 10.35 |
| 1/(BMP) = +4.20476 × 10−3 − 1.31145 × 10−5 × Reaction temp. − 4.36888 × 10−5 × Solid content − 9.28724 × 10−4 × H2O2 concent. − 4.17111 × 10−5 × Reaction time + 4.08924 × 10−7 × Reaction temp. × Solid content + 3.96470× 10−6 × Reaction temp. × H2O2 concent. − 8.50445 × 10−8 × Reaction temp. × Reaction time − 3.64937 × 10−5 × Solid content × H2O2 concent. − 2.27112 × 10−6 × Solid content × Reaction time + 4.32086 × 10−6 × H2O2 concent. × Reaction time + 1.02675 × 10−7 × Reaction temp.2 + 1.19229 × 10−5 × Solid content2 + 2.09008 × 10−4 × H2O2 concent.2 + 1.83088 × 10−6 × Reaction time2 | |||
| Wavelength (cm−1) | Region | 50 °C, 5% VS, 15 h, 2% H2O2 | 50 °C, 7% VS, 6 h, 1% H2O2 | 100 °C, 3% VS, 24 h, 3% H2O2 |
|---|---|---|---|---|
| 895–900 | Characteristic absorption peak of cellulose associated with the ß-glycosidic bond [19,20] | +++++ | + | ++++ |
| 1050 | C–O stretch of the C–O–C in cellulose, hemicellulose, and lignin [19,21] | +++++ | + | ++ |
| 1270 | C–O stretch in the guaiacyl aromatic ring associated with lignin [19,37] | +++ | ++ | +++++ |
| 1430–1460 | Aromatic skeletal vibration combined with C–H in plane deformation associated with lignin [37,38] | ++++ | +++ | +++++ |
| 1510–1600 | Aromatic skeletal vibration of lignin constituting conjugated C=C, aryl-substituted C=C, and alkenyl C=C stretch [37,38,39] | +++++ | + | +++ |
| 2920–2925 | C–H vibration of CH2 and CH3 groups [19,37] | +++ | ++ | +++++ |
| 3420 | Inter- and intramolecular hydrogen bonding [19] | ++++ | +++ | +++++ |
| 3446 | O–H stretch vibration in cellulose [37] | +++ | + | ++++ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perendeci, N.A.; Gökgöl, S.; Orhon, D. Impact of Alkaline H2O2 Pretreatment on Methane Generation Potential of Greenhouse Crop Waste under Anaerobic Conditions. Molecules 2018, 23, 1794. https://doi.org/10.3390/molecules23071794
Perendeci NA, Gökgöl S, Orhon D. Impact of Alkaline H2O2 Pretreatment on Methane Generation Potential of Greenhouse Crop Waste under Anaerobic Conditions. Molecules. 2018; 23(7):1794. https://doi.org/10.3390/molecules23071794
Chicago/Turabian StylePerendeci, N. Altınay, Sezen Gökgöl, and Derin Orhon. 2018. "Impact of Alkaline H2O2 Pretreatment on Methane Generation Potential of Greenhouse Crop Waste under Anaerobic Conditions" Molecules 23, no. 7: 1794. https://doi.org/10.3390/molecules23071794