Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopentenone Derivatives
Abstract
:1. Introduction
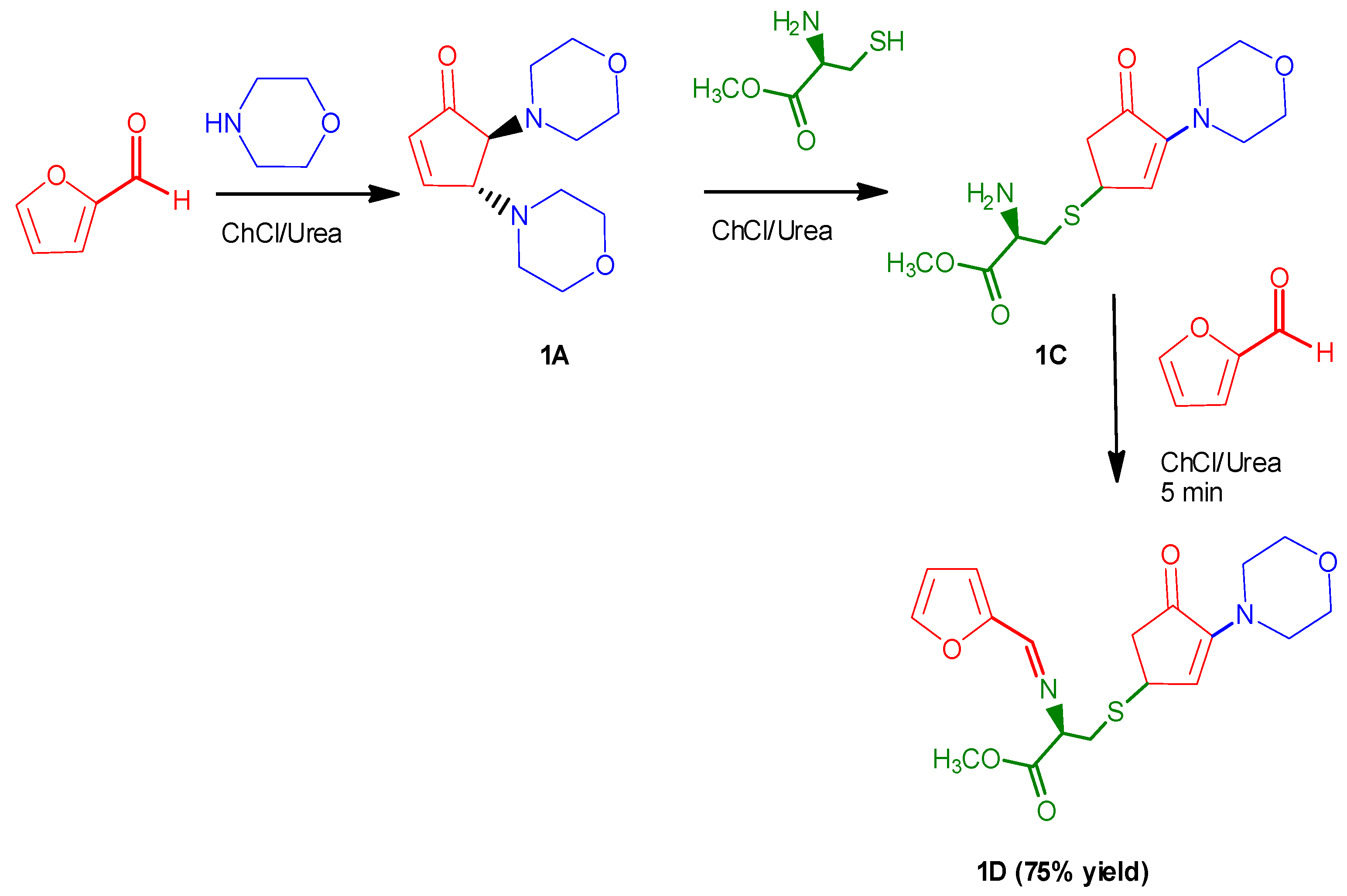
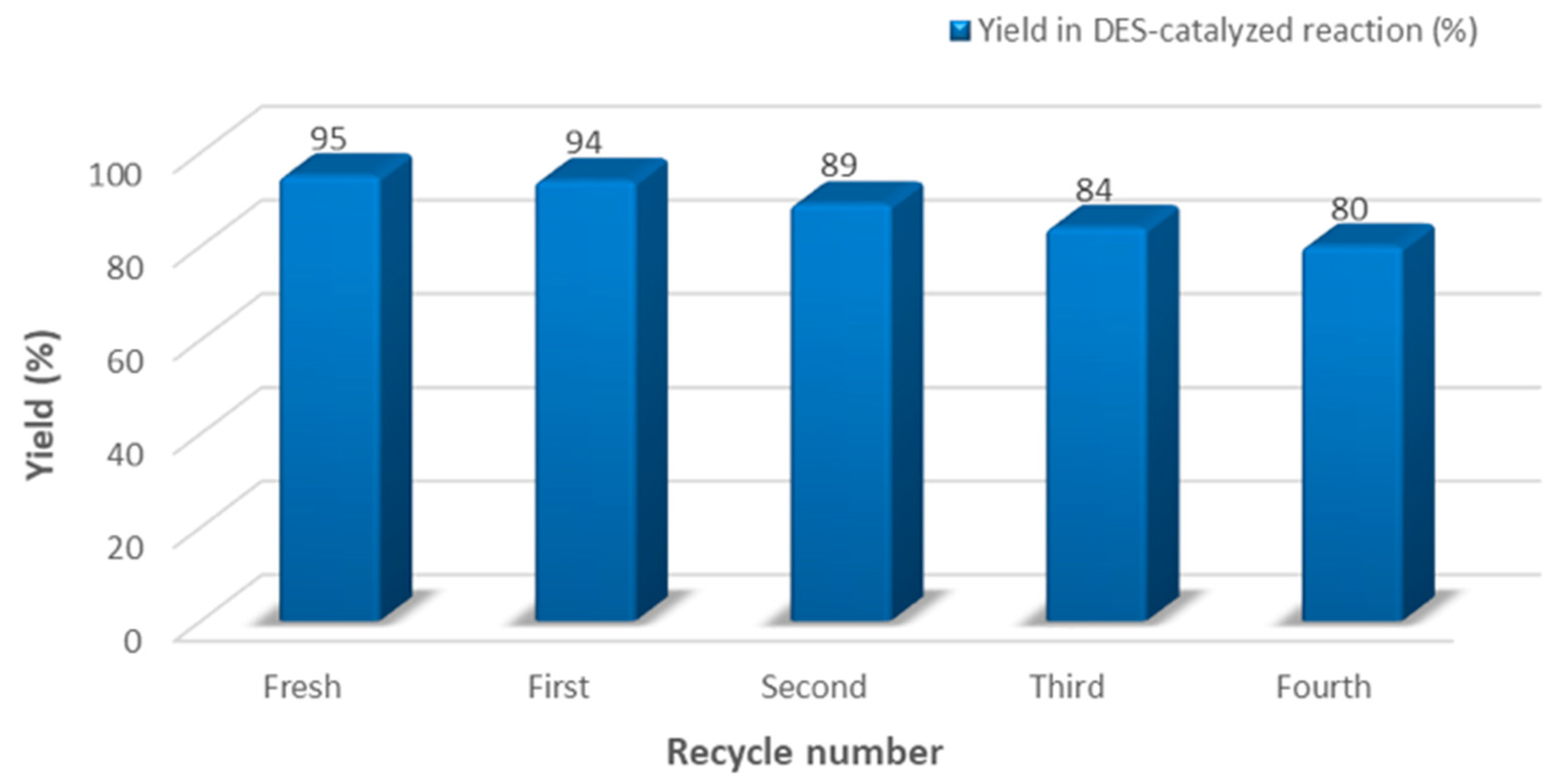
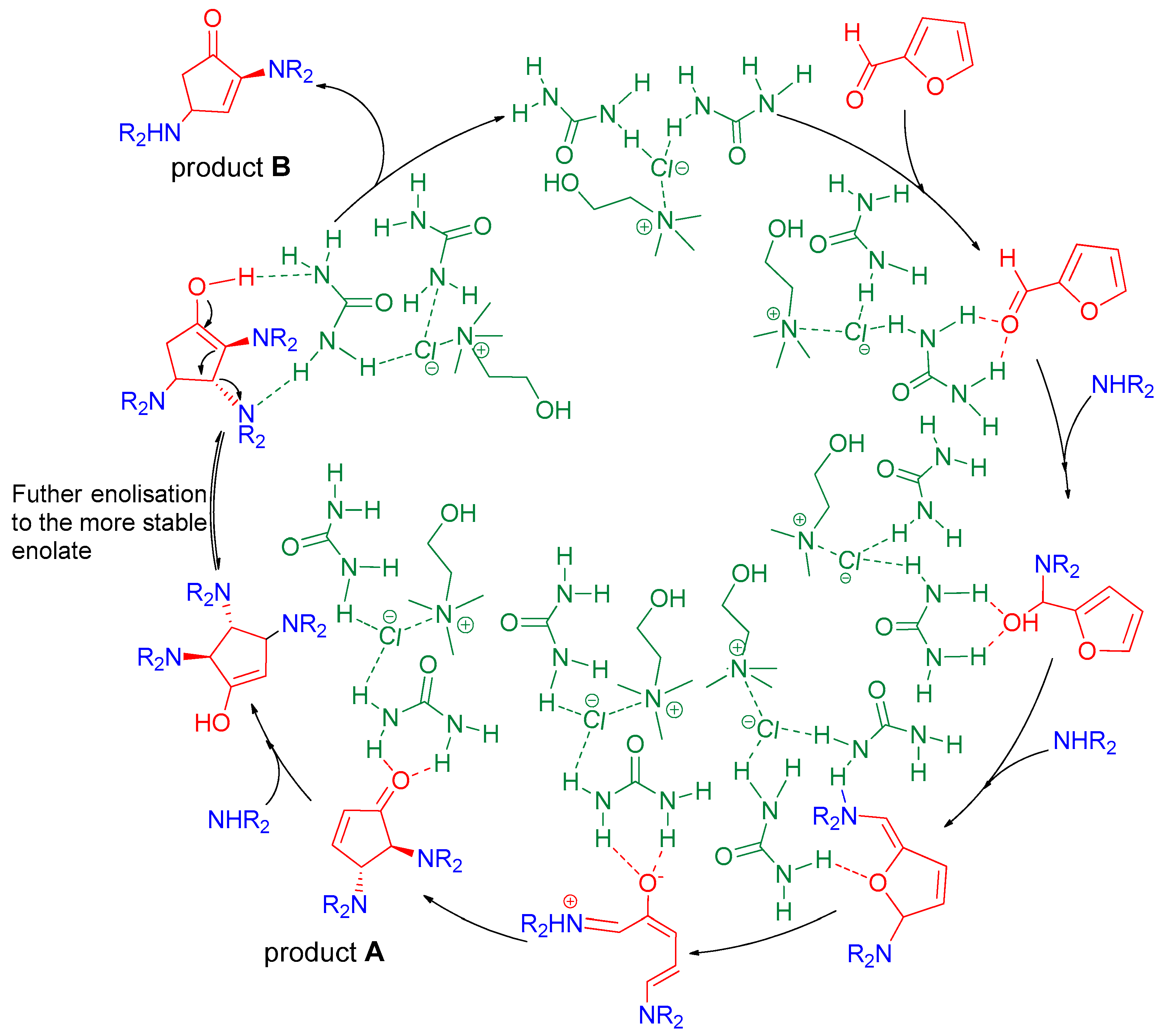
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Deep Eutectic Solvent Preparation
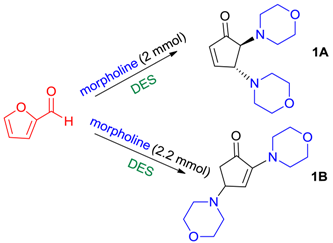
3.3. General Procedure for the Formation of 4,5-Difunctionalized Cyclopentenones (1A–12A)
3.4. General Procedure for the Formation of 2,4-Bifunctionalized Cyclopentenones (1B–2B)
3.5. Procedure for the Formation of Methyl (2R)-2-(furan-2- ylmethylene) amino)-3-((3-morpholino-4-oxocyclopent-2-en-1-yl)thio]propanoate (1D)
3.6. Recycling of Deep Eutectic Solvent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Peters, F.N. Industrial uses of furans. Ind. Eng. Chem. 1939, 31, 178–180. [Google Scholar] [CrossRef]
- Lukes, R.M.; Wilson, C.L. Reactions of furan compounds. XI. Side chain reactions of furfural and furfuryl alcohol over nickel-copper and iron-copper catalysts. J. Am. Chem. Soc. 1951, 73, 4790–4794. [Google Scholar] [CrossRef]
- Hashmi, S.K.; Wölfle, M.; Teles, J.H.; Frey, W. Bisphenols from furfurals by organocatalysis and gold catalysis. Synlett 2007, 11, 1747–1752. [Google Scholar] [CrossRef]
- Tšupova, S.; Rominger, F.; Rudolpha, M.; Hashmi, A.S.K. Synthesis of phenols from hydroxymethylfurfural (HMF). Green Chem. 2016, 18, 5800–5805. [Google Scholar] [CrossRef]
- Hashmi, S.K.; Rudolph, M.; Siehl, H.U.; Tanaka, M.; Bats, J.W.; Frey, W. Gold catalysis: Deuterated substrates as the key for an experimental insight into the mechanism and selectivity of the phenol synthesis. Chem. Eur. J. 2008, 14, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K.; Frost, T.M.; Bats, J.W. Highly selective gold-catalyzed arene synthesis. J. Am. Chem. Soc. 2000, 122, 11553–11554. [Google Scholar] [CrossRef]
- Takano, I.; Yasuda, I.; Nishijima, M.; Hitotsuyanagi, Y.; Takeya, K.; Itokawa, H. New cephalotaxus alkaloids from cephalotaxus harringtonia var. drupacea. J. Nat. Prod. 1996, 59, 965–967. [Google Scholar] [CrossRef]
- Inagaki, F.; Kinebuchi, M.; Miyakoshi, N.; Mukai, C. Formal Synthesis of (+)-Nakadomarin A. Org. Lett. 2010, 12, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- Magnus, P.; Fielding, M.R.; Wells, C.; Lynch, V. Stereoselective synthesis of the tricyclic core ABC-rings of nakadomarin and manzamine from a common intermediate. Tetrahedron Lett. 2002, 43, 947–950. [Google Scholar] [CrossRef]
- McGowan, J.C. The preparation of bases from the coloured compounds formed by condensation of furfuraldehyde with aromatic amines. J. Chem. Soc. 1949, 777–779. [Google Scholar] [CrossRef]
- Lewis, K.G.; Mulquiney, C.E. Aspects of the formation and use of stenhouse salts and related compounds. Tetrahedron 1977, 33, 463–475. [Google Scholar] [CrossRef]
- Lewis, K.G.; Mulquiney, C.E. Rearrangements in the furan series. ii. The reaction between furfuraldehyde and aromatic amines. Aust. J. Chem. 1979, 32, 1079–1092. [Google Scholar] [CrossRef]
- Hofmann, T. Characterization of the chemical structure of novel colored maillard reaction products from furan-2-carboxaldehyde and amino acids. J. Agric. Food Chem. 1998, 46, 932–940. [Google Scholar] [CrossRef]
- Li, S.W.; Batey, R.A. Mild lanthanide(III) catalyzed formation of 4,5-diaminocyclopent-2-enones from 2-furaldehyde and secondary amines: A domino condensation/ring-opening/electrocyclization process. Chem. Commun. 2007, 3759–3761. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) chloride in ethyl lactate as a smart ecofriendly system for efficient and rapid stereoselective synthesis of trans-4,5-diaminocyclopent-2-enones. ACS Sustain. Chem. Eng. 2013, 1, 541–544. [Google Scholar] [CrossRef]
- Nunes, J.P.M.; Afonso, C.A.M.; Caddick, S. Synthesis of 2,4-bifunctionalised cyclopentenones from 2-furaldehyde. RSC Adv. 2013, 3, 14975–14978. [Google Scholar] [CrossRef]
- Estevão, M.S.; Afonso, C.A.M. Synthesis of trans-4,5-diaminocyclopent-2-enones from furfural catalyzed by Er(III) immobilized on silica. Tetrahedron Lett. 2017, 58, 302–304. [Google Scholar] [CrossRef]
- Ramesh, D.; Reddy, T.S.; Narasimhulu, M.; Rajaram, S.; Suryakiran, N.; CMahesh, K.; Venkateswarlu, Y. Efficient and rapid stereoselective synthesis of trans-4,5-diaminocyclopent-2-enones by acidic ionic liquid under solvent-free conditions. Chem. Lett. 2009, 38, 586–587. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Messa, F.; Perrone, S.; Capua, M.; Tolomeo, F.; Troisi, L.; Capriati, V.; Salomone, A. Towards a sustainable synthesis of amides: Chemoselective palladium-catalysed aminocarbonylation of aryl iodides in deep eutectic solvents. Chem. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.F.A.; Esteves, N.R.; Coelho, J.A.S.; Afonso, C.A.M. Copper(II) triflate as a reusable catalyst for the synthesis of trans-4,5-diamino-cyclopent-2-enones in water. J. Org. Chem. 2018. [CrossRef] [PubMed]
- Lindström, U.M. Stereoselective Organic Reactions in Water. Chem. Rev. 2002, 10, 2751–2772. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Tagarelli, A.; Sindona, G. Simple and efficient MW-assisted cleavage of acetals and ketals in pure water. Tetrahedron Lett. 2007, 48, 8623–8627. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Rosati, O. Highly efficient and versatile chemoselective addition of amines to epoxides in water catalyzed by erbium(III) triflate. Tetrahedron Lett. 2008, 49, 2289–2293. [Google Scholar] [CrossRef]
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective acetylation of small biomolecules and their derivatives catalyzed by Er(OTf)3. Catalysts 2017, 7, 269. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Davies, D.L.; Capper, G.; Rasheed, R.K.; Tambyrajah, V. Ionic Liquids and Their Use As solvents. U.S. Patent 7,183,433, 27 February 2007. [Google Scholar]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 10, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.; Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutierrez, M.C.; Ferrer, M.L. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. ChemSusChem 2014, 7, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, J. Deep eutectic mixtures: Promising sustainable solvents for metal-catalysed and metal-mediated organic reactions. Eur. J. Inorg. Chem. 2015, 31, 5147–5157. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 4, 612–632. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/ catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Wang, Q.; Du, H.; Zhang, L. Deep eutectic solvent-based microwave-assisted method for extraction of hydrophilic and hydrophobic components from radix salviae miltiorrhizae. Molecules 2016, 21, 1383. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lyu, G.; Liu, Y.; Lou, R.; Lucia, L.A.; Yang, G.; Chen, J.; Saeed, H.A.M. Deep eutectic solvents (DESs) for the isolation of willow lignin (salix matsudana cv. zhuliu). Int. J. Mol. Sci. 2017, 18, 2266. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L.; Perna, F.M.; Logrieco, A.; Capriati, V.; Solfrizzo, M. Deep Eutectic Solvents as novel and effective extraction media for quantitative determination of ochratoxin a in wheat and derived products. Molecules 2017, 22, 121. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 0-19-850234-6. [Google Scholar]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK′s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Prat, D.; Haylerb, J.; Wellsc, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehadad, S.; Dunne, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Desai, K.R. Green Chemistry Microwave Synthesis, 1st ed.; Himalaya Publication House: New Delhi, India, 2005; p. 20. [Google Scholar]
- Kappe, O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, D.; Kappe, O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Calandruccio, C.; Salerno, R.; Procopio, A. Tunable microwave-assisted method for the solvent-free and catalyst-free peracetylation of natural products, Beilstein. J. Org. Chem. 2016, 12, 2222–2233. [Google Scholar]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, L.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Delso, I.; Tallarida, M.A.; De Nino, A. Nitrones and nucleobase-containing spiro-isoxazolidines derived from isatin and indanone: Solvent-free microwave-assisted stereoselective synthesis and theoretical calculations. RSC Adv. 2017, 7, 48980–48988. [Google Scholar] [CrossRef]
- Mingos, D.M.P. Microwave in chemical syntheses. Chem. Ind. 1994, 596–599. [Google Scholar]
- De la Hoz, A.; Díaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Dalpozzo, R.; De Nino, A.; Nardi, M.; Russo, B.; Procopio, A. Erbium(III) triflate: A valuable catalyst for the synthesis of aldimines, ketimines, and enaminones. Synthesis 2006, 31, 1127–1132. [Google Scholar] [CrossRef]
- Nardi, M.; Cozza, A.; De Nino, A.; Oliverio, M.; Procopio, A. One-pot synthesis of dibenzo[b,e][1,4]diazepin-1-ones. Synthesis 2012, 44, 800–804. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. Facile ecofriendly synthesis of monastrol and its structural isomers via biginelli reaction. ACS Sustain. Chem. Eng. 2014, 2, 1228–1233. [Google Scholar] [CrossRef]
- Nardi, M.; Oliverio, M.; Costanzo, P.; Sindona, G.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135. [Google Scholar] [CrossRef]
- Molnar, M.; Komar, M.; Brahmbhatt, H.; Babić, J.; Jokić, S.; Rastija, V. Deep Eutectic Solvents as convenient media for synthesis of novel coumarinyl schiff bases and their QSAR studies. Molecules 2017, 22, 1482. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Di Gioia, M.L.; Leggio, A.; Liguori, A.; Viscomi, M.C. Deprotection of N-Nosyl-α-amino Acids by using solid-supported mercaptoacetic acid. Eur. J. Org. Chem. 2009, 22, 3795–3800. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Barattucci, A.; Bonaccorsi, P.; Leggio, A.; Minuti, L.; Romio, E.; Temperini, A.; Siciliano, C. Deprotection/reprotection of the amino group in α-amino acids and peptides. A one-pot procedure in [Bmim][BF4] ionic liquid. RSC Adv. 2014, 4, 2678–2686. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Gagliardi, A.; Leggio, A.; Leotta, V.; Romio, E.; Liguori, A. N-Urethane protection of amines and amino acids in an ionic liquid. RSC Adv. 2015, 5, 63407–63420. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Aqueous MW eco-friendly protocol for amino group protection. RSC Adv. 2015, 5, 18751–18760. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, M.L.; Leggio, A.; Le Pera, A.; Siciliano, C.; Sindona, G.; Liguori, A. An efficient and highly selective deprotection of N-Fmoc-a-amino acid and lipophilic N-Fmocdipeptide methyl esters with aluminium trichloride and N,N-dimethylaniline. J. Pept. Res. 2004, 63, 383–387. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Di Gioia, M.L.; Leggio, A.; Liguori, A.; Perri, F.; Siciliano, C.; Viscomi, M.C. A new non-natural arginine-like amino acid derivative with a sulfamoyl group in the side-chain. Amino Acids 2010, 38, 691–700. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Di Gioia, M.L.; Liguori, A.; Perri, F.; Siciliano, C.; Spinella, M. N-Alkylation of N-Arylsulfonyl-α-amino Acid Methyl Esters by Trialkyloxonium Tetrafluoroborates. Tetrahedron 2011, 67, 9708–9714. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Belsito, E.L.; Leggio, A.; Leotta, V.; Romio, E.; Siciliano, C.; Liguori, A. Reduction of amide carbonyl group and formation of modified amino acids and dipeptides. Tetrahedron Lett. 2015, 56, 2062–2066. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Leggio, A.; Malagrinò, F.; Romio, E.; Siciliano, C.; Liguori, A. N-Methylated α-Amino Acids And Peptides: Synthesis And Biological Activity. Mini-Rev. Med. Chem. 2016, 16, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M.; Cano, N.H.; De Nino, A.; Di Gioia, M.L.; Maiuolo, L.; Oliverio, M.; Santiago, A.; Sorrentino, D.; Procopio, A. An eco-friendly tandem tosylation/Ferrier N-glycosylation of amines catalyzed by Er(OTf)3 in 2-MeTHF. Tetrahedron Lett. 2017, 58, 1721–1726. [Google Scholar] [CrossRef]
- Yadav, U.N.; Shankarling, G.S. Room temperature ionic liquid choline chloride–oxalic acid: A versatile catalyst for acid-catalyzed transformation in organic reactions. J. Mol. Liq. 2014, 191, 137–141. [Google Scholar] [CrossRef]
- Capua, M.; Perrone, S.; Perna, F.M.; Vitale, P.; Troisi, L.; Salomone, A.; Capriati, V. An Expeditious and Greener Synthesis of 2-Aminoimidazoles in deep eutectic solvents. Molecules 2016, 21, 924. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Hooshmand, S.E. Choline chloride/urea as a deep eutectic solvent/organocatalyst promoted three-component synthesis of 3-aminoimidazo-fused heterocycles via Groebke–Blackburn–Bienayme process. Tetrahedron Lett. 2016, 57, 310–313. [Google Scholar] [CrossRef]
- Dilauro, G.; Cicco, L.; Perna, F.M.; Vitale, P.; Capriati, V. Solvent-catalyzed umpolung carbonesulfur bond-forming reactions by nucleophilic addition of thiolate and sulfinate ions to in situ derived nitrosoalkenes in deep eutectic solvents. C. R. Chim. 2017, 20, 617–623. [Google Scholar] [CrossRef]
Sample Availability: samples of the compounds 1–5, dry preserved, are available from the authors. |

| Entry | DES (Molar Ratio) | Temp (°C) | Time (min) | Product | Yield 3 (%) |
|---|---|---|---|---|---|
| 1 1 | ChCl:glycerol (1:2) | rt | 60 | 1A | - |
| 2 1 | ChCl:glycerol (1:2) | 80 | 5 | 1A | 20 |
| 3 1 | ChCl:citric acid (1:1) | 80 | 5 | 1A | 35 |
| 4 1 | ChCl:lactic acid (1:1) | 80 | 5 | 1A | 45 |
| 5 1 | ChCl:urea (1:2) | rt | 60 | 1A | - |
| 6 1 | ChCl:urea (1:2) | 60 | 5 | 1A | 95 |
| 7 1 | ChCl:urea (1:2) | 80 | 5 | 1A | 95 |
| 8 2 | ChCl:urea (1:2) | 60 | 5 | 1B | 0 4 |
| 9 2 | ChCl:urea (1:2) | 60 | 240 | 1B | 98 |
| 10 2 | ChCl:urea (1:2) | 60 (MW) | 30 | 1B | 45 5 |
| 11 2 | ChCl:urea (1:2) | 60 (MW) | 60 | 1B | 45 5 |

| Entry | Amine | Products | Yields b (%) | |
| 1 | 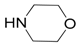 | 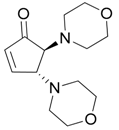 | 1A | 9595 |
| 2 | 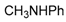 |  | 2A | 90 |
| 3 |  | 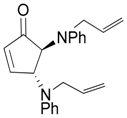 | 3A | 92 |
| 4 |  | 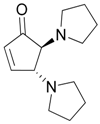 | 4A | 95 |
| 5 | 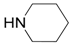 |  | 5A | 96 |
| 6 | 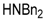 | 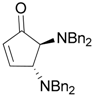 | 6A | 89 |
| 7 |  | 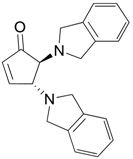 | 7A | 91 |
| 8 | 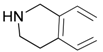 | 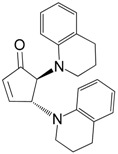 | 8A | 93 |
| 9 | 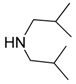 |  | 9A | 94 |
| 10 | 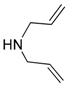 |  | 10A | 94 |
| 11 | 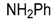 |  | 11A | 40 c |
| 12 | 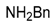 | 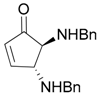 | 12A | 0 d |
| 13 |  | 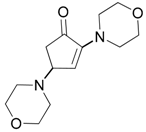 | 1B | 98 |
| 14 |  | 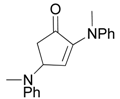 | 2B | 94 |
| 15 |  | 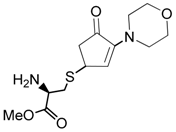 | 1C | 85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gioia, M.L.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopentenone Derivatives. Molecules 2018, 23, 1891. https://doi.org/10.3390/molecules23081891
Di Gioia ML, Nardi M, Costanzo P, De Nino A, Maiuolo L, Oliverio M, Procopio A. Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopentenone Derivatives. Molecules. 2018; 23(8):1891. https://doi.org/10.3390/molecules23081891
Chicago/Turabian StyleDi Gioia, Maria Luisa, Monica Nardi, Paola Costanzo, Antonio De Nino, Loredana Maiuolo, Manuela Oliverio, and Antonio Procopio. 2018. "Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopentenone Derivatives" Molecules 23, no. 8: 1891. https://doi.org/10.3390/molecules23081891








