Selective Extraction and Antioxidant Properties of Thiol-Containing Peptides in Soy Glycinine Hydrolysates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reduction of Disulfide Bonds in SGHs
2.2. Optimization of the Extraction Conditions of TCPs
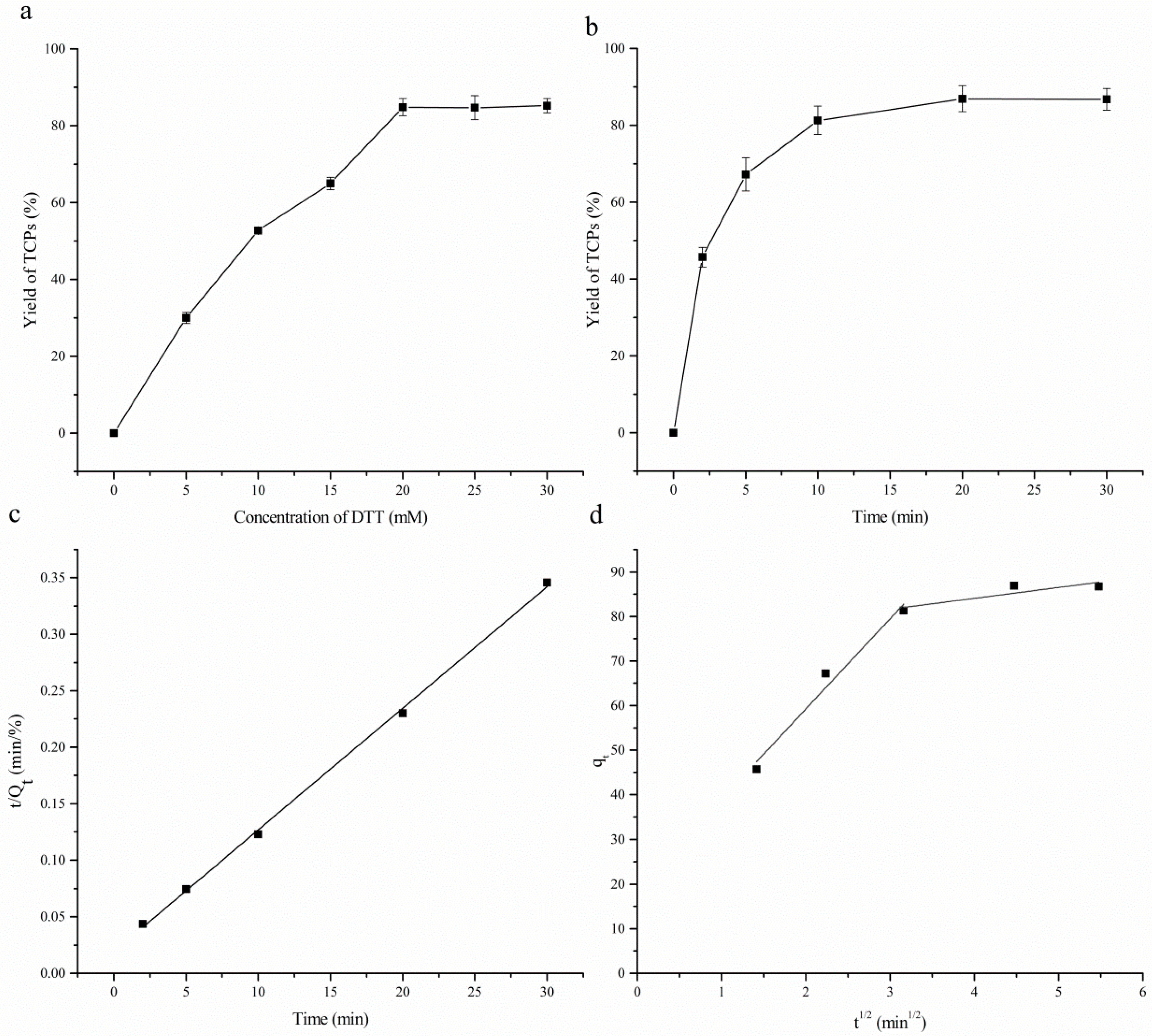
2.2.1. Optimization of the Capture Conditions of TCPs
2.2.2. Optimization of the Desorption Conditions of TCPs
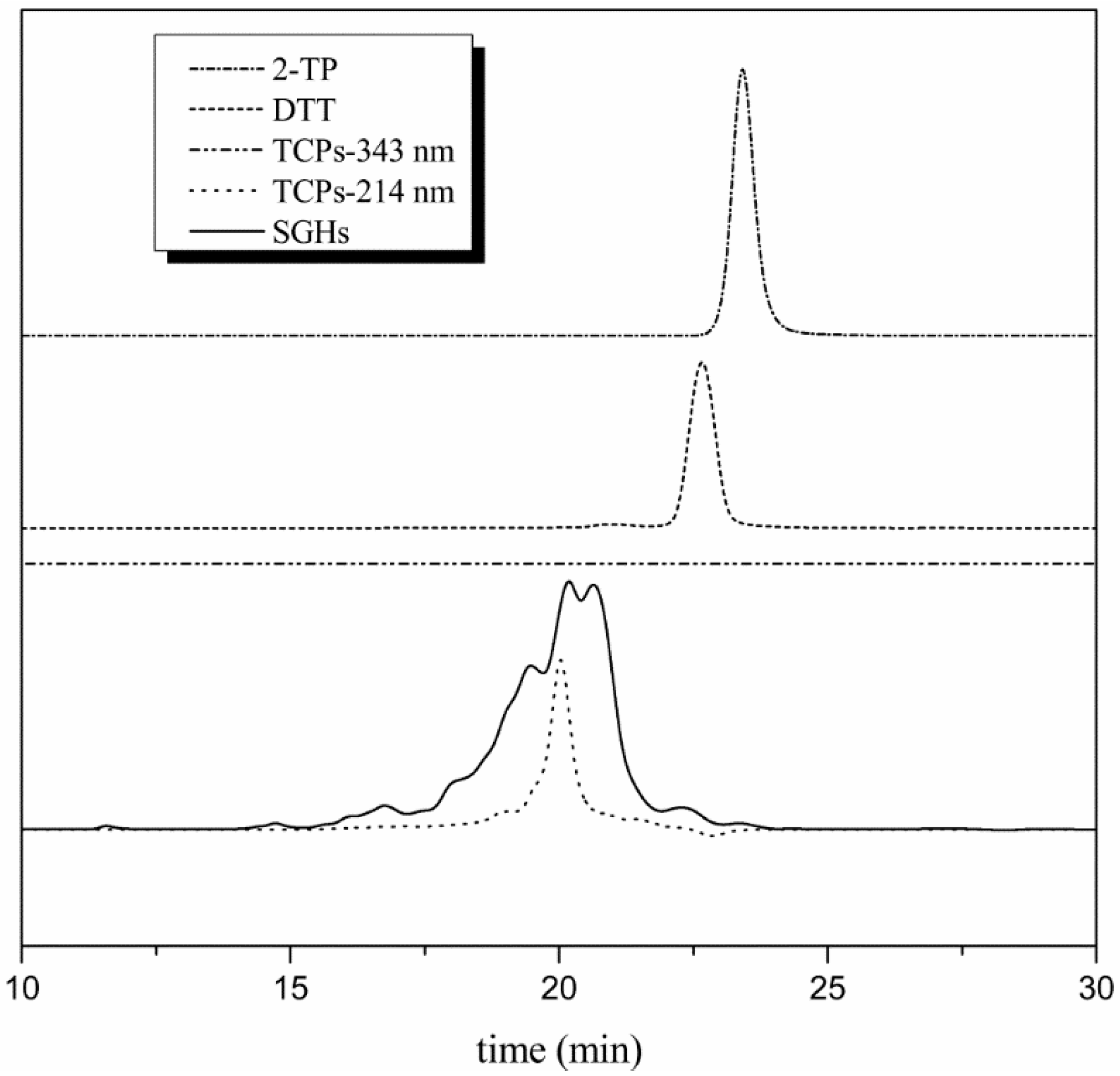
2.2.3. Purification of TCPs by Reversed Phase Chromatography Column
2.3. Specificity to TCPs
2.4. Antioxidant Activities of TCPs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Soy Glycinin (11 S)
3.3. Enzymatic Hydrolysis of 11 S
3.4. Reduction of Disulfide Bonds in Soy Glycinine Hydrolysates (SGHs)
3.5. TCPs Enrichment from Reduced SGHs
3.6. Reversed Phase Chromatography
3.7. Sulfhydryl Group Content Measurement
3.8. Size-Exclusion High Performance Chromatography (SEC-HPLC)
3.9. Alkylation of Sulfhydryl Groups and Matrix-Assisted Laser Desorption/Ionization—Time-of-Flight Mass Spectrometry (MALDI-TOF-MS)
3.10. Antioxidant Activities of TCPs
3.10.1. Scavenging Activity against DPPH Radical
3.10.2. Scavenging Activity against Hydroxyl Radical
3.10.3. Superoxide Anion Scavenging Activity Assay
3.10.4. Reducing Power Measurement
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, M.; Wehr, C.; Schade, J.; MacGregor, J. Inactivation of aflatoxin B1 mutagenicity by thiols. Food Chem. Toxicol. 1982, 20, 887–892. [Google Scholar] [CrossRef]
- Duh, P.D.; Wu, S.C.; Chang, L.W.; Chu, H.L.; Yen, W.J.; Wang, B.S. Effects of three biological thiols on antimutagenic and antioxidant enzyme activities. Food Chem. 2009, 114, 87–92. [Google Scholar] [CrossRef]
- Anonymous. Mechanism of toxicity of lysinoalanine. Nutr. Rev. 1989, 47, 362–364. [Google Scholar]
- Friedman, M.; Gumbmann, M.R.; Grosjean, O. Nutritional improvement of soy flour. J. Nutr. 1984, 114, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Peter, H.; Bolt, H. Effect of potential antidotes on the acute toxicity of acrylonitrile. Int. Arch. Occup. Environ. Health 1981, 49, 157–163. [Google Scholar] [CrossRef]
- Loguercio, C.; Taranto, D.; Beneduce, F.; del Vecchio Blanco, C.; De Vincentiis, A.; Nardi, G.; Romano, M. Glutathione prevents ethanol induced gastric mucosal damage and depletion of sulfhydryl compounds in humans. Gut 1993, 34, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.D. In Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990, 10, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Geneve, J.; Degott, C.; Letteron, P.; Tinel, M.; Descatoire, V.; Larrey, D.; Amouyal, G.; Pessayre, D. Metabolic activation of the tricyclic antidepressant amineptine—II: Protective role of glutathione against in vitro and in vivo covalent binding. Biochem. Pharmacol. 1987, 36, 331–337. [Google Scholar] [CrossRef]
- Trickier, D.; Shklar, G.; Schwartz, J. Inhibition of oral carcinogenesis by glutathione. Nutr. Cancer 1993, 20, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.; Chen, W.; Mulchandani, A.; Mehra, R.K. Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol. Bioeng. 2000, 70, 518–524. [Google Scholar] [CrossRef]
- Han, C.H.; Liu, J.C.; Fang, S.U.; Hou, W.C. Antioxidant activities of the synthesized thiol-contained peptides derived from computer-aided pepsin hydrolysis of yam tuber storage protein, dioscorin. Food Chem. 2013, 138, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhao, M.; Li, W.; You, L.; Wang, J.; Wang, H.; Ren, J. Chemical and cellular antioxidant activity of two novel peptides designed based on glutathione structure. Food Chem. Toxicol. 2012, 50, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Chen, H.J.; Huang, S.S.; Liao, J.C.; Hou, W.C.; Lin, Y.H. Defensin protein from sweet potato (Ipomoea batatas [L.] Lam ‘Tainong 57’) storage roots exhibits antioxidant activities in vitro and ex vivo. Food Chem. 2012, 135, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Improvement in the safety of foods by sulfhydryl-containing amino acids and peptides. A review. J. Agric. Food Chem. 1994, 42, 3–20. [Google Scholar] [CrossRef]
- Tsumura, K.; Saito, T.; Tsuge, K.; Ashida, H.; Kugimiya, W.; Inouye, K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT-Food Sci. Technol. 2005, 38, 255–261. [Google Scholar] [CrossRef]
- Liu, C.F.; Pan, T.M. Beneficial Effects of Bioactive Peptides Derived from Soybean on Human Health and Their Production by Genetic Engineering; Intech Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Panizzolo, L.A.; Añón, M.C. Foaming properties of soy protein isolate hydrolysates. J. Food Nutr. Sci. 2015, 3, 1–9. [Google Scholar]
- Murota, I.; Taguchi, S.; Sato, N.; Park, E.Y.; Nakamura, Y.; Sato, K. Identification of antihyperuricemic peptides in the proteolytic digest of shark cartilage water extract using in vivo activity-guided fractionation. J. Agric. Food Chem. 2014, 62, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Karametsi, K.; Kokkinidou, S.; Ronningen, I.; Peterson, D.G. Identification of bitter peptides in aged cheddar cheese. J. Agric. Food Chem. 2014, 62, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Sun, C.; Zhao, Y.; Xiong, L.; Sun, Q. Purification and identification of antioxidant peptides from peanut protein isolate hydrolysates using UHR-Q-TOF mass spectrometer. Food Chem. 2014, 161, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, S.; Cai, X.; Hong, J.; Wang, S. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods 2014, 10, 46–53. [Google Scholar] [CrossRef]
- Haber, E.; Anfinsen, C.B. Regeneration of enzyme activity by air oxidation of reduced subtilisin-modified ribonuclease. J. Biol. Chem. 1961, 236, 422–424. [Google Scholar] [PubMed]
- Wang, H.; Qian, W.-J.; Chin, M.H.; Petyuk, V.A.; Barry, R.C.; Liu, T.; Gritsenko, M.A.; Mottaz, H.M.; Moore, R.J.; Camp, D.G. Characterization of the Mouse Brain Proteome Using Global Proteomic Analysis Complemented with Cysteinyl-Peptide Enrichment. J. Proteome Res. 2006, 5, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qian, W.J.; Chen, W.N.U.; Jacobs, J.M.; Moore, R.J.; Anderson, D.J.; Gritsenko, M.A.; Monroe, M.E.; Thrall, B.D.; Camp, D.G. Improved proteome coverage by using high efficiency cysteinyl peptide enrichment: The human mammary epithelial cell proteome. Proteomics 2005, 5, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulech, J.; Solis, N.; Edwards, A.V.; Puckeridge, M.; White, M.Y.; Cordwell, S.J. Large-scale capture of peptides containing reversibly oxidized cysteines by thiol-disulfide exchange applied to the myocardial redox proteome. Anal. Chem. 2013, 85, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hua, Y.; Chen, Y.; Zhang, C.; Kong, X. Heavy metal complexation of thiol-containing peptides from soy glycinin hydrolysates. Int. J. Mol. Sci. 2015, 16, 8040–8058. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.J. Sulfhydryl content of glycinin—Effect of reducing agents. J. Agric. Food Chem. 1993, 41, 168–176. [Google Scholar] [CrossRef]
- Hansen, R.E.; Winther, J.R. An introduction to methods for analyzing thiols and disulfides: Reactions, reagents, and practical considerations. Anal. Biochem. 2009, 394, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Norder, H. Covalent chromatography as a means of isolating thiol peptides from large proteins: Application to human ceruloplasmin. J. Chromatogr. A 1981, 215, 341–350. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Doğan, M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater. 2007, 101, 388–396. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Khang, D.; Dung, T.; Elzaawely, A.; Xuan, T. Phenolic Profiles and Antioxidant Activity of Germinated Legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Shia, Z.; Yao, Y.; Zhu, Y.; Ren, G. Nutritional composition and antioxidant activity of twenty mung bean cultivars in China. Crop J. 2016, 4, 398–406. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Sharma, A. Antioxidant and Ace Inhibitory Properties of Poultry Viscera Protein Hydrolysate and Its Peptide Fractions. J. Food Biochem. 2012, 36, 494–501. [Google Scholar] [CrossRef]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014, 150, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chi, Y.J.; Zhao, M.Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant activity of enzymatic hydrolysates from eggshell membrane proteins and its protective capacity in human intestinal epithelial Caco-2 cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Jung, W.-K.; Kim, S.-K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Riener, C.K.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4, 4′-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Pick Number | IAM | NEM | Distance in m/z | Content of Cys |
|---|---|---|---|---|
| 1 | 198.774 | 198.769 | −0.005 | 0 |
| 2 | 274.156 | 274.149 | −0.007 | 0 |
| 3 | 284.22 | 284.222 | 0.002 | 0 |
| 4 | 312.92 | 312.911 | −0.009 | 0 |
| 5 | 318.22 | 318.202 | −0.018 | 0 |
| 6 | 833.497 | 833.4 | −0.097 | 0 |
| 7 | 905.439 | 905.408 | −0.032 | 0 |
| 8 | 992.494 | 992.485 | −0.009 | 0 |
| 9 | 1169.603 | 1169.569 | −0.033 | 0 |
| 10 | 249.849 | 318.202 | 68.353 | 1 |
| 11 | 315.021 | 383.135 | 68.113 | 1 |
| 12 | 335.026 | 403.133 | 68.106 | 1 |
| 13 | 482.176 | 550.227 | 68.051 | 1 |
| 14 | 517.18 | 585.244 | 68.064 | 1 |
| 15 | 559.205 | 627.266 | 68.061 | 1 |
| 16 | 713.328 | 781.374 | 68.046 | 1 |
| 17 | 765.341 | 833.408 | 68.068 | 1 |
| 18 | 777.363 | 845.422 | 68.059 | 1 |
| 19 | 799.351 | 867.417 | 68.066 | 1 |
| 20 | 815.341 | 883.404 | 68.063 | 1 |
| 21 | 821.341 | 889.402 | 68.061 | 1 |
| 22 | 864.424 | 932.471 | 68.047 | 1 |
| 23 | 910.428 | 978.458 | 68.031 | 1 |
| 24 | 921.453 | 989.523 | 68.069 | 1 |
| 25 | 928.422 | 996.486 | 68.064 | 1 |
| 26 | 940.452 | 1008.478 | 68.026 | 1 |
| 27 | 992.494 | 1060.551 | 68.056 | 1 |
| 28 | 1041.518 | 1109.589 | 68.071 | 1 |
| 29 | 1049.587 | 1117.515 | 67.928 | 1 |
| 30 | 1063.511 | 1131.587 | 68.076 | 1 |
| 31 | 1079.487 | 1147.567 | 68.081 | 1 |
| 32 | 1085.519 | 1153.529 | 68.01 | 1 |
| 33 | 1150.572 | 1218.581 | 68.009 | 1 |
| 34 | 1174.575 | 1242.592 | 68.017 | 1 |
| 35 | 1177.58 | 1245.652 | 68.072 | 1 |
| 36 | 1188.58 | 1256.645 | 68.065 | 1 |
| 37 | 1197.631 | 1265.685 | 68.055 | 1 |
| 38 | 1204.609 | 1272.617 | 68.008 | 1 |
| 39 | 1210.61 | 1278.59 | 67.981 | 1 |
| 40 | 1226.607 | 1294.613 | 68.006 | 1 |
| 41 | 1249.618 | 1317.626 | 68.008 | 1 |
| 42 | 713.328 | 849.39 | 136.063 | 2 |
| 43 | 921.453 | 1057.523 | 136.07 | 2 |
| 44 | 1049.587 | 1185.595 | 136.008 | 2 |
| 45 | 1125.593 | 1261.639 | 136.046 | 2 |
| TCPs | Glutathione | |
|---|---|---|
| DPPH (mg/mL) A | 0.10 ± 0.004 a | 0.03 ± 0.001 b |
| Hydroxyl radical (mg/mL) | 1.49 ± 0.07 a | 0.36 ± 0.014 b |
| Superoxide anion radical (mg/mL) | 0.084 ± 0.004 a | 0.052 ± 0.003 b |
| Reducing power B | 0.38 ± 0.01 a | 0.57 ± 0.031 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Kong, X.; Chen, Y.; Zhang, C.; Hua, Y.; Li, X. Selective Extraction and Antioxidant Properties of Thiol-Containing Peptides in Soy Glycinine Hydrolysates. Molecules 2018, 23, 1909. https://doi.org/10.3390/molecules23081909
Ding X, Kong X, Chen Y, Zhang C, Hua Y, Li X. Selective Extraction and Antioxidant Properties of Thiol-Containing Peptides in Soy Glycinine Hydrolysates. Molecules. 2018; 23(8):1909. https://doi.org/10.3390/molecules23081909
Chicago/Turabian StyleDing, Xiuzhen, Xiangzhen Kong, Yeming Chen, Caimeng Zhang, Yufei Hua, and Xiangyang Li. 2018. "Selective Extraction and Antioxidant Properties of Thiol-Containing Peptides in Soy Glycinine Hydrolysates" Molecules 23, no. 8: 1909. https://doi.org/10.3390/molecules23081909





