Molecular Level Structure of Biodegradable Poly(Delta-Valerolactone) Obtained in the Presence of Boric Acid
Abstract
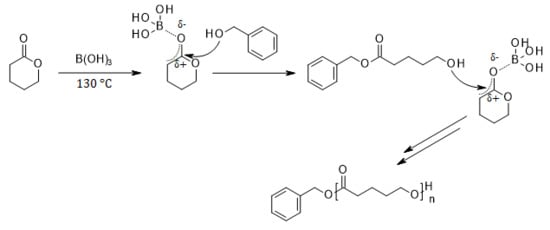
:1. Introduction
2. Results and Discussion
2.1. The 1H-NMR Studies
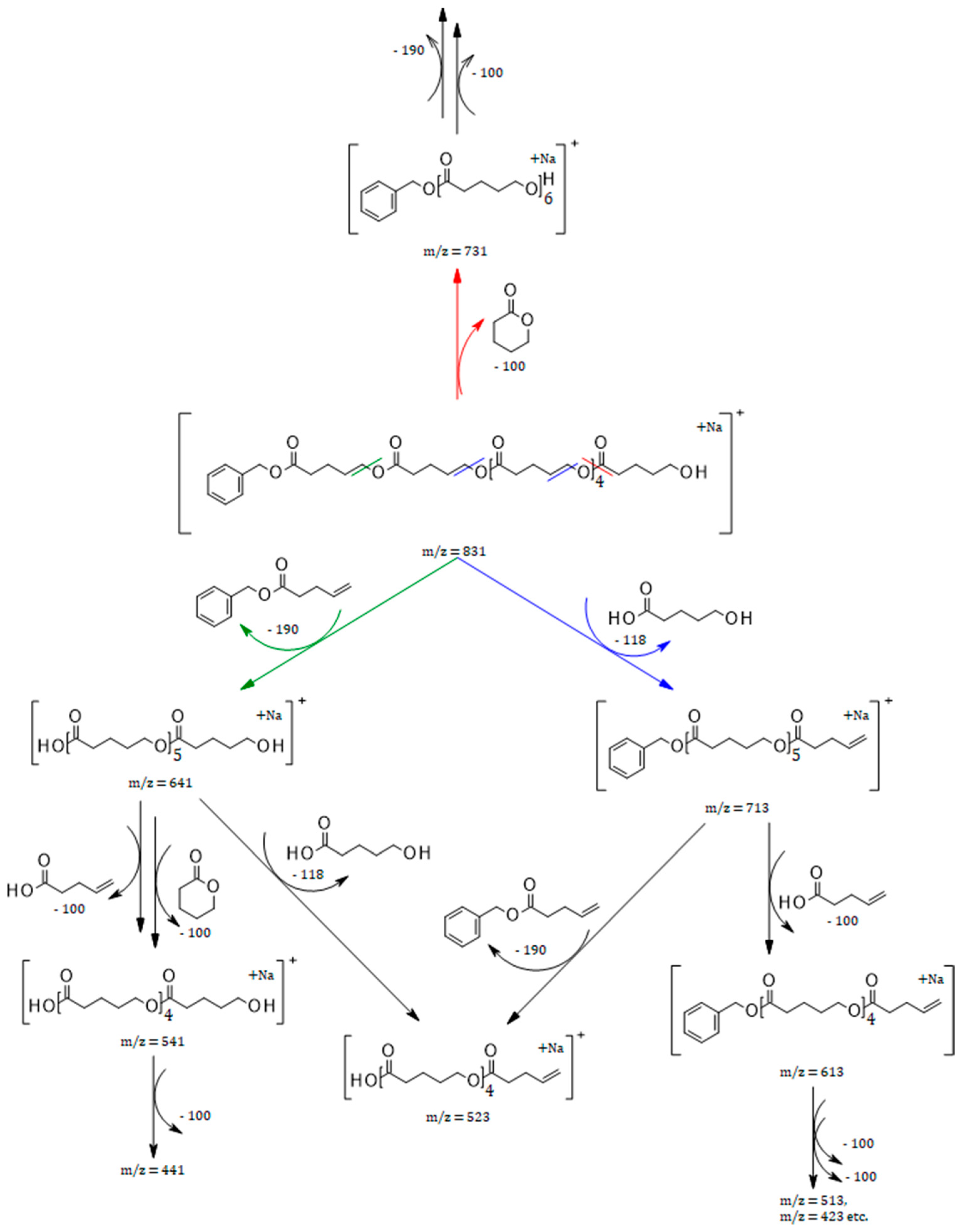
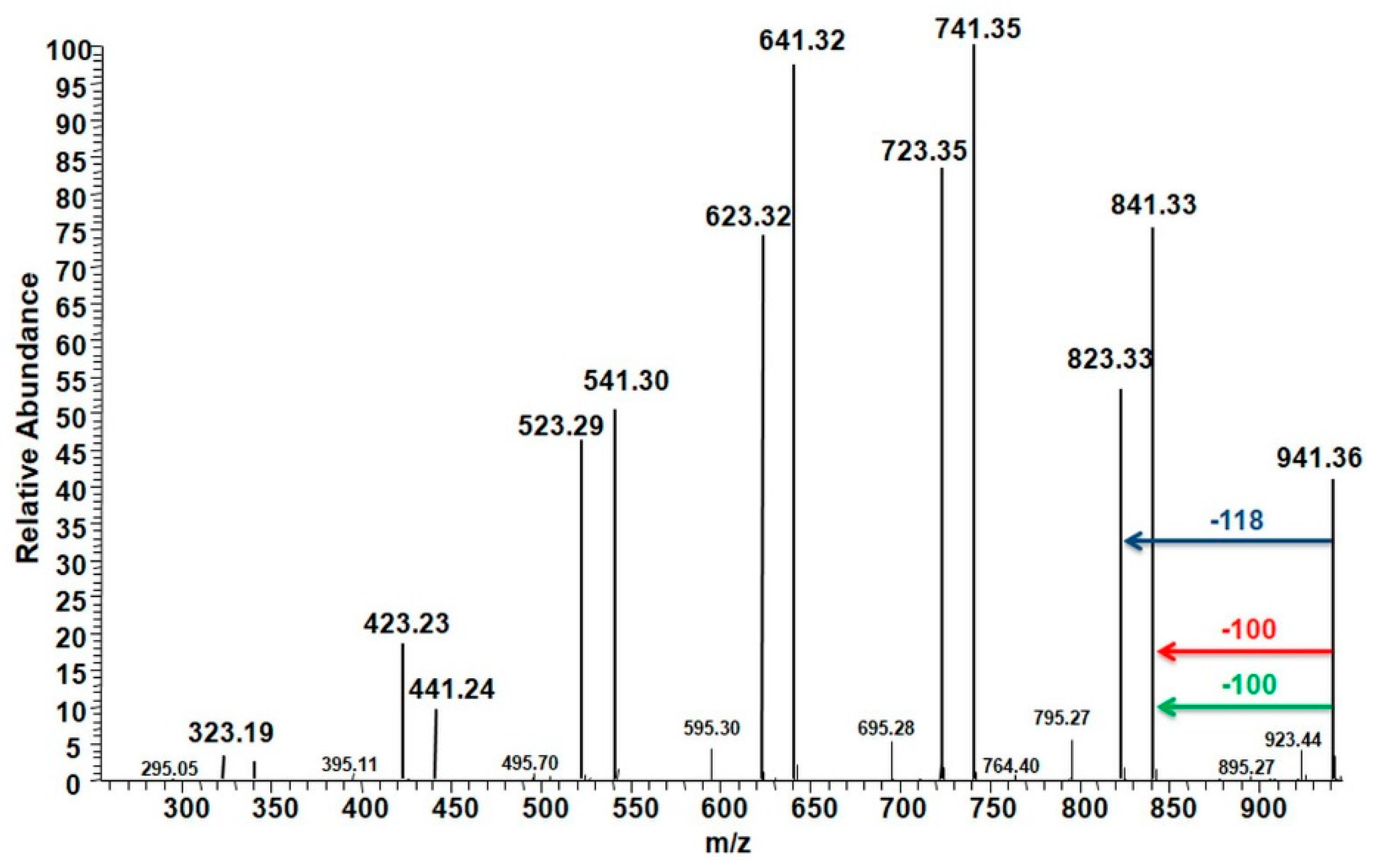
2.2. ESI-MS Analysis
2.3. MALDI TOF-MS
2.4. ESI-MS Negative Ion Analysis
3. Materials and Methods
3.1. Gel Permeation Chromatography (GPC)
3.2. Proton Nuclear Magnetic Resonance (1HNMR)
3.3. Electrospray Ionization Mass Spectrometry (ESI-MSn) Analysis
3.4. Matrix Assisted Laser Desorption Ionization (MALDI-TOF-MS)
3.5. Homopolymerization of δ-Valerolactone
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albertsson, A.-C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, Y.; Huang, H.; Liu, L.; Chen, Y. Well-Defined Poly (α-amino-δ-valerolactone) via Living Ring-Opening Polymerization. Macromolecules 2018, 51, 2526–2532. [Google Scholar] [CrossRef]
- Nakayama, A.; Kawasaki, N.; Arvanitoyannis, I.; Iyoda, J.; Yamamoto, N. Synthesis and degradability of a novel aliphatic polyester: Poly (β-methyl-δ-valerolactone-co-l-lactide). Polymer 1995, 36, 1295–1301. [Google Scholar] [CrossRef]
- Li, S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J. Biomed. Mater. Res. 1999, 48, 342–353. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Albertsson, A. Heterogeneous biodegradation of polycaprolactone–low molecular weight products and surface changes. Macromol. Chem. Phys. 2002, 203, 1357–1363. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, Z.; Leng, X.; Wang, Y.; Li, Y. Boric acid as biocatalyst for living ring-opening polymerization of ε-caprolactone. Polymer 2015, 78, 51–58. [Google Scholar] [CrossRef]
- Lin, W. Comparison of thermal characteristics and degradation properties of ϵ-caprolactone copolymers. J. Biomed. Mater. Res. 1999, 47, 420–423. [Google Scholar] [CrossRef]
- Nakayama, A.; Kawasaki, N.; Maeda, Y.; Arvanitoyannis, I.; Aiba, S.; Yamamoto, N. Study of biodegradability of poly (δ-valerolactone-co-l-lactide) s. J. Appl. Polym. Sci. 1997, 66, 741–748. [Google Scholar] [CrossRef]
- Hakkarainen, M. Aliphatic polyesters: Abiotic and biotic degradation and degradation products. In DeGradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002; pp. 113–138. ISBN 978-3-540-45734-3. [Google Scholar]
- Hakkarainen, M.; Höglund, A.; Odelius, K.; Albertsson, A.-C. Tuning the release rate of acidic degradation products through macromolecular design of caprolactone-based copolymers. J. Am. Chem. Soc. 2007, 129, 6308–6312. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Yang, H.; Deri, F.; Ko, Y. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Luef, K.P.; Stelzer, F.; Wiesbrock, F. Poly(hydroxy alkanoate) s in medical applications. Chem. Biochem. Eng. Q. 2015, 29, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Chan-Thaw, C.E.; Marelli, M.; Psaro, R.; Ravasio, N.; Zaccheria, F. New generation biofuels: γ-valerolactone into valeric esters in one pot. RSC Adv. 2013, 3, 1302–1306. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chu, I.-M. Methoxy poly(ethylene glycol)-b-poly(valerolactone) diblock polymeric micelles for enhanced encapsulation and protection of camptothecin. Eur. Polym. J. 2008, 44, 3922–3930. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Gr. Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Faÿ, F.; Renard, E.; Langlois, V.; Linossier, I.; Vallée-Rehel, K. Development of poly (ε-caprolactone-co-l-lactide) and poly (ε-caprolactone-co-δ-valerolactone) as new degradable binder used for antifouling paint. Eur. Polym. J. 2007, 43, 4800–4813. [Google Scholar] [CrossRef]
- Deng, P.; Teng, F.; Zhou, F.; Song, Z.; Meng, N.; Feng, R. Methoxy poly (ethylene glycol)-b-poly (δ-valerolactone) copolymeric micelles for improved skin delivery of ketoconazole. J. Biomater. Sci. Polym. Ed. 2017, 28, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zeng, F.; Dunne, M.; Allen, C. Methoxy poly(ethylene glycol)-block-poly(δ-valerolactone) copolymer micelles for formulation of hydrophobic drugs. Biomacromolecules 2005, 6, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Jagadeeshan, S.; Nair, S.A.; Kumar, G.V. Evaluation of triblock copolymeric micelles of δ-valerolactone and poly(ethylene glycol) as a competent vector for doxorubicin delivery against cancer. J. Nanobiotechnol. 2011, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F. Doxorubicin: Anticancer Antibiotics; Academic Press: Cambridge, MA, USA, 2012; ISBN 0-323-13973-6. [Google Scholar]
- Nielen, M.W. MALDI time-of-flight mass spectrometry of synthetic polymers. Mass Spectrom. Rev. 1999, 18, 309–344. [Google Scholar] [CrossRef]
- Adamus, G.; Hakkarainen, M.; Höglund, A.; Kowalczuk, M.; Albertsson, A.-C. MALDI-TOF MS Reveals the Molecular Level Structures of Different Hydrophilic-Hydrophobic Polyether-esters. Biomacromolecules 2009, 10, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, M. New vistas in mass spectrometry for sequence analysis of natural and synthetic biodegradable macromolecules. Chim. Oggi-Chem. Today 2016, 34, 12–15. [Google Scholar]
- Altuntaş, E.; Schubert, U.S. “Polymeromics”: Mass spectrometry based strategies in polymer science toward complete sequencing approaches: A review. Anal. Chim. Acta 2014, 808, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, M.; Adamus, G. Mass spectrometry for the elucidation of the subtle molecular structure of biodegradable polymers and their degradation products. Mass Spectrum. Rev. 2016, 35, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Rizzarelli, P.; Montaudo, M.S.; Kowalczuk, M.; Montaudo, G. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry with size-exclusion chromatographic fractionation for structural characterization of synthetic aliphatic copolyesters. Rapid Commun. Mass Spectrum. 2006, 20, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Gruendling, T.; Weidner, S.; Falkenhagen, J.; Barner-Kowollik, C. Mass spectrometry in polymer chemistry: A state-of-the-art up-date. Polym. Chem. 2010, 1, 599–617. [Google Scholar] [CrossRef]
- Adamus, G.; Kwiecień, I.; Maksymiak, M.; Bałakier, T.; Jurczak, J.; Kowalczuk, M. Molecular level structure of novel synthetic analogues of aliphatic biopolyesters as revealed by multistage mass spectrometry. Anal. Chim. Acta 2014, 808, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Wesdemiotis, C.; Solak, N.; Polce, M.J.; Dabney, D.E.; Chaicharoen, K.; Katzenmeyer, B.C. Fragmentation pathways of polymer ions. Mass Spectrum. Rev. 2011, 30, 523–559. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G. Molecular level structure of (R,S)-3-hydroxybutyrate/(R,S)-3-hydroxy-4-ethoxybutyrate copolyesters with dissimilar architecture. Macromolecules 2009, 42, 4547–4557. [Google Scholar] [CrossRef]
- De Winter, J.; Lemaur, V.; Marsal, P.; Coulembier, O.; Cornil, J.; Dubois, P.; Gerbaux, P. Mechanistic study of the collision-induced dissociation of sodium-cationized polylactide oligomers: A joint experimental and theoretical investigation. J. Am. Soc. Mass Spectrom. 2010, 21, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedliński, Z.; Juzwa, M.; Adamus, G.; Kowalczuk, M.; Montaudo, M. Anionic polymerization of pentadecanolide. A new route to a potentially biodegradable aliphatic polyester. Macromol. Chem. Phys. 1996, 197, 2923–2929. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| Sample | Mn (g/mol−1) | Mw/Mn |
|---|---|---|
| PVL1 | 1500 | 1.5 |
| PVL2 | 4500 | 1.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duale, K.; Zięba, M.; Chaber, P.; Di Fouque, D.J.; Memboeuf, A.; Peptu, C.; Radecka, I.; Kowalczuk, M.; Adamus, G. Molecular Level Structure of Biodegradable Poly(Delta-Valerolactone) Obtained in the Presence of Boric Acid. Molecules 2018, 23, 2034. https://doi.org/10.3390/molecules23082034
Duale K, Zięba M, Chaber P, Di Fouque DJ, Memboeuf A, Peptu C, Radecka I, Kowalczuk M, Adamus G. Molecular Level Structure of Biodegradable Poly(Delta-Valerolactone) Obtained in the Presence of Boric Acid. Molecules. 2018; 23(8):2034. https://doi.org/10.3390/molecules23082034
Chicago/Turabian StyleDuale, Khadar, Magdalena Zięba, Paweł Chaber, Dany Jeanne Di Fouque, Antony Memboeuf, Cristian Peptu, Iza Radecka, Marek Kowalczuk, and Grażyna Adamus. 2018. "Molecular Level Structure of Biodegradable Poly(Delta-Valerolactone) Obtained in the Presence of Boric Acid" Molecules 23, no. 8: 2034. https://doi.org/10.3390/molecules23082034