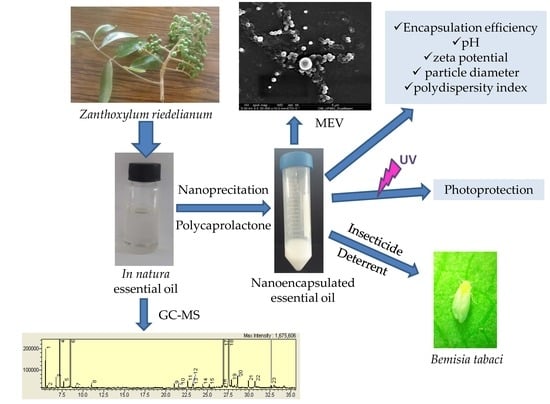
2.3. Physicochemical Characterization of Nanospheres
The nanoprecipitation method used to encapsulate the
Z. riedelianum fruit essential oils was based on precipitation of the polymer after addition of a nonsolvent to a solution containing the polymer. Four mechanisms occurred, namely, supersaturation, nucleation, growth by condensation, and growth by coagulation, leading to the formation of nanoparticles [
31]. Those authors also noted that this method shows good reproducibility in the laboratory and constitutes a good strategy for nanoparticle production at an industrial scale. Other advantages offered by the method include the simple execution and the use of solvents relatively less toxic than many others, such as ethanol and acetone [
32].
The nanoprecipitation method was efficient in encapsulating
Z. riedelianum fruit essential oils since the colloidal suspensions exhibited EE% ≥ 98.66% (
Table 3). Previous studies reported similar results using the same method and the PCL polymer; for instance, EE% was ~99% for rosemary essential oil [
33] and greater than 96% for
Z. rhoifolium leaf essential oil [
34]. Forim et al. [
23] used a new, nanoprecipitation-based method capable of encapsulating 100% of neem extract.
The stability of the nanoparticulate systems was maintained by employing a high hydrophilic–lipophilic balance (HLB) surfactant—Tween
®80—which has the purpose of preventing particle coalescence and diffusion of the encapsulated active substance. The low HLB surfactant, Span
®60, present in the organic phase, is necessary to obtain a population of nanospheres with a small and homogeneous size [
35,
36]. The stability and uniformity of the suspensions were evaluated by their pH, zeta potential (ZP), particle diameter (PD), and polydispersity index (PdI) values.
The pH values of the formulations decreased with increasing amounts of essential oil (
Table 3) but remained between 6.19 and 6.66, a range considered satisfactory [
37]. Previous studies on the encapsulation of plant extracts and essential oils using PCL as a polymer revealed pH values between 4 and 6, corroborating our results [
23,
34]. Low pH values (<4.0) indicate degradation of the polymer and/or of some sample component. The polymer itself (PCL) can decrease pH values due to the relaxation of its polymer chains, resulting in the exposure of carboxylic acid groups. However, acidic pH values can lead to polymer hydrolysis, yielding unstable suspensions and generating sediments in sample vials [
37]. No sediment formation was observed before any analysis or bioassay was conducted, indicating the stability of the nanoformulations, which was also confirmed by the zeta potential (ZP) values.
The ZP (ζ) measures the electrostatic repulsion of the particles’ electric charges. ZP values ≥30 mV or ≤−30 mV indicate good physicochemical stability of the suspension, since the repulsion between charged particles is higher, preventing coalescence driven by occasional collisions between adjacent nanoparticles [
38,
39,
40]. In the present study, no significant difference existed between the ZP values of the samples (
Table 3), with values around −24 mV; the sole exception was NS2, whose ZP was −19.0 ± 2.4 mV. Similar results were obtained in the encapsulation of rosemary essential oil, with ZP ≤ −19.9 [
33]. PCL nanospheres containing
Z. rhoifolium leaf essential oil presented ZP ≤ −26.12 [
34].
According to Woodruff and Hutmacher [
26], nanosphere size can vary between 10 and 1000 nm. All suspensions in the present study presented nanospheres within this diameter range, with values between 106.7 and 129.2 nm (
Table 3). A significant difference existed in particle diameter values; however, the increase in the amount of essential oil (mg) did not influence this feature. According to Schaffazick et al. [
40], average nanoparticle diameters fall between 100 and 300 nm regardless of the preparation method (e.g., solvent displacement (nanoprecipitation), emulsion polymerization, or emulsification-diffusion), thus corroborating our results. Similar results were also obtained by Pinto et al. [
41], who reported a mean diameter of 173.6 nm for PCL nanocapsules containing
Lippia sidoides leaf essential oil formed by solvent emulsification-diffusion. Nanocapsules containing rosemary essential oil formed by nanoprecipitation showed PDs of approximately 220 nm [
33]. Yang et al. [
42] obtained nanoparticles containing garlic essential oil with approximately 233 nm in diameter using the dispersion-fusion technique.
Another important parameter in the evaluation of suspension quality is the polydispersity index (PdI), which provides information on the size homogeneity of suspended nanoparticles. Some researchers classify PdI values up to 0.3 as monodispersed [
33,
43,
44]. No significant difference existed in the PdI values, with values close to 0.2 (
Table 3). These results indicate a narrow range of nanosphere sizes.
2.6. Deterrence of EO and NSEO Z. riedelianum against B. tabaci
In all bioassays with nymphs and adults, it was not observed phytotoxicity by the essential oil on bean leaves, even at higher concentrations of 1% and 1.5%.
The deterrent effect of the essential oil against adult whiteflies was evaluated through oviposition on bean leaves in free-choice and no-choice tests.
In the free-choice test, all tested concentrations of EO and NSEO significantly reduced the number of eggs relative to the controls with Tween and empty nanospheres (formulation without the essential oil), respectively (
Table 4). The reduction in the number of eggs with both forms of the essential oil was similar to that of the insecticide treatment (
Table 4). The number of eggs did not differ between NSEO and EO treatments over the tested concentration range. The data suggest that the nanoencapsulation process did not affect the whitefly oviposition-deterrent effect of
Z. riedelianum. According to the oviposition index, the NSEO and EO and the insecticide spiromesifen were whitefly oviposition deterrents at all tested doses (
Table 4).
In the no-choice test, the number of eggs was significantly lower in the treatment with EO at all doses than the control (Tween) (
Table 4). Moreover, all tested doses of the EO were oviposition-deterring according to the oviposition index (
Table 4). NSEO reduced the number of eggs only in treatments at 0.25% and 1.5% compared to the NS control (empty nanosphere—formulation without essential oil). The oviposition-deterrent effect of NSEO was observed only at the 0.25% concentration (
Table 4). Similar to the free-choice test, the no-choice test showed no differences in the number of eggs between NSEO and EO at all concentrations tested. Similar results were observed by Costa et al. [
22]. The
Z. riedelianum fruit essential oil decreased the number of
B. tabaci eggs on tomato leaves by 85.7% and 94.2% for the 1.0% and 1.5% concentrations, respectively. Christofoli et al. [
34] also demonstrated that
Z. rhoifolium Lam. leaf EO at 0.5% or 1.0% reduced
B. tabaci oviposition by 71% and 77%, respectively, three days after treatment application. These authors also showed that the
Z. rhoifolium nanoencapsulated essential oil at the 2% and 5% doses reduced the number of eggs by 95.6% and 93.9%, respectively, when compared to the control (water). The greater oviposition reduction obtained with
Zanthoxylum essential oil by Christofoli et al. [
34] compared to the present study may stem from the variation in the chemical composition of the essential oil. The quality and quantity of essential oil components may vary according to plant genetics, climate, soil composition, plant organ, age, and stage of the vegetative cycle, etc. [
30,
49,
50,
51].
The reduction in whitefly oviposition on bean leaves treated with
Z. riedelianum NSEO and EO may have been caused by different factors. These factors include the lipidic nature of the essential oil, which may have affected a glue-like substance secreted by whiteflies used to attach the egg pedicel to the host plant tissue [
52,
53] and prevented the eggs from attaching to the plant, causing them to fall. Yang et al. [
16] also showed a decrease in the number of eggs deposited by female whiteflies on tomato leaves treated with
Thymus vulgaris L.,
Pogostemon cablin (Blanco) Benth., and
Corymbia citriodora essential oils.
Saad et al. [
54] attributed the decrease in
B. tabaci oviposition to the volatile compounds found in citronella (
Cymbopogon nardus L.) essential oil, which may have affected the development of the female whitefly, reducing its reproductive capacity. An example of this activity was reported by Rao et al. [
55], who found that
Dysdercus koenigii nymphs treated with
Artemisia annua essential oil showed ovarian development issues, decreasing the number of produced oocytes with a consequent influence on egg production.
Frequent changes in feeding sites due to the persistence of the essential oil in the plant tissue are another factor that may have influenced whitefly oviposition. This may reduce phloem sap suction, leading to a decrease in the number of mature eggs for deposition [
16,
56]. Reduced feeding and oviposition can also be influenced by the detection of some volatile substances by chemoreceptors present in the insect’s tarsi, deterring oviposition [
52,
57].
2.7. Mortality of Second-Instar B. tabaci Nymphs
In the first experiment, EO and NSEO
Z. riedelianum had a significant effect on the mortality of nymphs (56.53–85.20% and 30.23–66.18%, respectively) (
Figure 3). Nymphal mortality gradually increased with essential oil concentrations, regardless of form. A log-logistic model provided the best fit for both EO and NSEO (
R2 = 89.80%) (
Figure 3). When the two curves were compared using Wilcoxon’s test, the essential oils were significantly different (
p < 0.001).
A lower activity of NSEO was also observed by Carvalho et al. [
59]. PCL nanoformulations containing neem oil killed 18.9% of 1st-instar
B. tabaci nymphs, compared to 60% caused by the free form. In turn, Carvalho et al. [
60] obtained different results for two types of nanocapsules containing Azamax
® 1.2 EC, a commercial formulation enriched with azadirachtin and 3-tigloylazadirachtol. For the same concentration of Azamax, PCL, and poly-β-hydroxybutyrate (PHB) nanocapsules caused the mortality of 98% and 70.6% of
B. tabaci nymphs, respectively. This variation in results was attributed to environmental conditions, such as greater photoperiod, light intensity, or thermal amplitudes. These factors may have accelerated polymer breakdown, with a concomitant faster release of the active compound.
The lower activity of the NSEO compared to EO forms may also be related to the type of formulation used for nanoparticle preparation and/or to the environmental conditions. According to Soppimath et al. [
24], the release of active compounds by nanoparticles, as well as nanoparticle biodegradation, is important for a successful formulation. Release rates depend on the desorption of the active principle from the surface of the nanoparticles, their diffusion through the polymer matrix, erosion of the matrix, and erosion-associated diffusion. Therefore, diffusion and biodegradation are essential processes for the controlled release system. Depending on the formulation, the active compounds may remain entrapped in the polymer matrix, reducing product activity. De Oliveira et al. [
61] prepared
Lippia sidoides essential oil-based nanoparticles with different ratios of alginate (ALG) and cashew gum (CG): 1:3, 1:1, and 3:1. The nanoparticles formed with a 3:1 ALG:CG ratio exhibited a lower, more controlled release of the essential oil (45% in 50 h), whereas an ALG:CG 1:3 ratio released 65% of the essential oils after 5 h. Thus, the nanoparticle release profile depended on the ALG:CG ratio.
The second experiment was conducted with the same nanoformulations but at a lower relative humidity (48.41 ± 7.78%). Nymphal mortality also increased with increasing concentrations of essential oil (
Figure 4).
According to Wilcoxon’s non-parametric test, the two curves were significantly different (
p < 0.001), where the EO killed more 2nd-instar nymphs than the NSEO. Although this finding corroborates the first experiment, the efficiency of the NSEO at the 1.5% dose increased in the second test (
p < 0.05). The EO form of
Z. riedelianum fruit essential oil at concentrations of 0.25, 0.5, 1.0, and 1.5% caused mortality of 55.57, 64.30, 86.98, and 91.23% of the nymphs, respectively. The NSEO at concentrations of 1.0% and 1.5% killed 70.55% and 82.87% of the nymphs, respectively. The lowest concentrations, 0.25% and 0.5%, did not differ significantly, reducing nymph numbers by just 30.93% and 31.20%, respectively. The highest dose (1.5%) of both NSEO and EO were statistically similar to the chemical insecticide cyantraniliprole (
Table 5).
The higher efficiency of the essential oil in the second experiment is likely related to the environmental conditions, such as the lower relative humidity, corroborating Carvalho et al. [
60]. Many essential oil components are photounstable and thermolabile [
48]. These characteristics limit the use of essential oils in agriculture due to a high exposure to UV radiation and temperature. However, nanoencapsulation improves the efficiency and persistence of these natural products [
62]. Therefore, although the percent nymphal mortality was higher in free essential oil form, nanoencapsulates may become more advantageous by reducing photo- and thermo-degradation issues of the active compound, possibly maintaining their activity for longer periods of time [
23].
Nanoencapsulation protects the active compounds in the essential oil from environmental factors (e.g., light and heat) that affect their molecular structures. UV radiation with wavelength (λ) near 350 nm is capable of breaking bonds between carbon atoms in organic molecules, thereby generating radicals that accelerate the oxidation rate of the compounds [
63]. This effect can be minimized or retarded by coating polymers such as PCL, as these will act as a protective barrier, leading to a slow release of the compounds adsorbed into their polymer matrix as they degrade.
Other studies have also demonstrated the efficiency of nanoparticles containing plant extracts in pest control. Forim et al. [
23] used a new technique to prepare nanocapsules containing
Azadirachta indica (neem), based on the solvent displacement method. These nanocapsules caused the mortality of 100% of
Plutella xylostella larvae. The nanoparticles containing
Allium sativum L. essential oil controlled
Tribolium castaneum (red flour beetles) with >80% efficacy, whereas the free form reduced the beetles by only 11% after five months of product application [
42]. Nanoemulsions of three species of the genus
Achillea also presented insecticidal activity against
T. castaneum [
64].
Some hypotheses can be formulated to explain nymphal susceptibility to
Z. riedelianum essential oil: (1) Whiteflies, for instance, possess a waxy layer on their body, which hinders insecticide penetration [
65]. Essential oils thus have the advantage of disrupting this layer and acting on insect metabolism in several ways, including inducing mitochondrial membrane depolarization [
8]. The decrease in membrane potential (depolarization) affects the calcium ion cycle and reduces the pH gradient, affecting the proton pump and ATP pool [
7,
66,
67]. These changes render the mitochondrial membrane abnormally permeable, which results in oxidative stress and bioenergetic failure, leading to necrosis- and apoptosis-induced cell death [
68]. (2) In insects, acetylcholinesterase (AChE) inhibition and acetylcholine accumulation leads to excitation and death [
69]. Some essential oil active compounds, such as monoterpenes, are competitive inhibitors of acetylcholinesterase (AchE). The inhibition mechanism may be related to the chemical structure of the monoterpene, comprising one methyl allyl group and a double bond [
70]. Limonene, one of the major components of the
Z. riedelianum fruit essential oil, inhibited 87.42% of the AchE activity [
71]. The compounds α-pinene and β-caryophyllene—alone or in combination—also inhibited AchE [
72]. Moreover, monoterpenes can act lethally by interfering with the neuromodulator octopamine or the GABA-bound chloride channels [
73]. This feature grants essential oils a selective toxicity against insects since mammals (vertebrates) do not have octopamine receptors. (3) The insecticidal activity of the
Z. riedelianum fruit essential oil can also be attributed to the presence of compounds such as limonene and β-myrcene. Hollingsworth [
74] showed that treatments containing 1% limonene caused 99% mortality of
Aleurodicus dispersus whiteflies. Five mg/cm
2 of β-myrcene induced 100% mortality of
Blatella germanica adult females [
75]. Prieto et al. [
76] attributed the insecticidal activity of
Z. rhoifolium against
Sitophilus oryzae to β-myrcene and β-phellandrene.
In our study, twenty-two compounds were identified in
Z. riedelianum fruit essential oil (
Table 1). Probably, the biological effects of this essential oil was from the synergism between all molecules, from the major molecules, or be modulated by the minor molecules as observed in others studies with essential oils [
7,
77]. Zarrad et al. [
71] reported that
Citrus aurantium L. essential oil was more toxic to
B. tabaci adults than pure limonene. This result revealed the additive or synergistic effect of the components of
C. aurantium oil. The combination of different substances has the advantage of reducing the resistance of insect pests, as they decrease selection pressure, which would not occur if a single compound was used. These factors reinforce the advantages of using essential oils compared to the isolated component.
In summary, the
Z. riedelianum fruit essential oil was effective as an insecticide and oviposition deterrent against
B. tabaci. Although some results have showed that the EO killed more second-instar nymphs than the NSEO form, it presented similar activity to the synthetic insecticide cyantraniliprole. The nanoencapsulated form demonstrated similar efficiency to the EO form in the free-choice and no-choice tests for whitefly oviposition deterrence. Nanoencapsulated essential oils offer a greater advantage over the free essential oil forms, with protection conferred by the PCL polymer against photodegradation (results described herein). Through the nanoencapsulation, it is possible to optimize the maintenance of the essential oil in the field reducing the requirement of extra applications that clearly impact final cost of the crops. Given that the nanoformulated products usually display differential bioavailability in the field, a greater stability implies a greater permanence in the cultivation [
47,
78]. Moreover, once essential oils are volatiles, they are easily spread in the environment, losing their ability to control insects, so we need a way for them to be fixed in the field. Thus, nanoformulation may be an option to solve this sort of practical issue. Therefore, the results presented here may be further used as the foundation to the development of commercial products using essential oils such as from fruits of
Z. riedelianum.