Immobilized and Free Cells of Geotrichum candidum for Asymmetric Reduction of Ketones: Stability and Recyclability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Immobilized Cells
2.1.1. FTIR Spectroscopy
2.1.2. SEM Spectroscopy
2.2. Biocatalytic Activity of Free and Immobilized G. candidum AS 2.361 Cells
2.3. Stability
2.3.1. pH Tolerance
2.3.2. Thermostability
2.3.3. Storage Stability
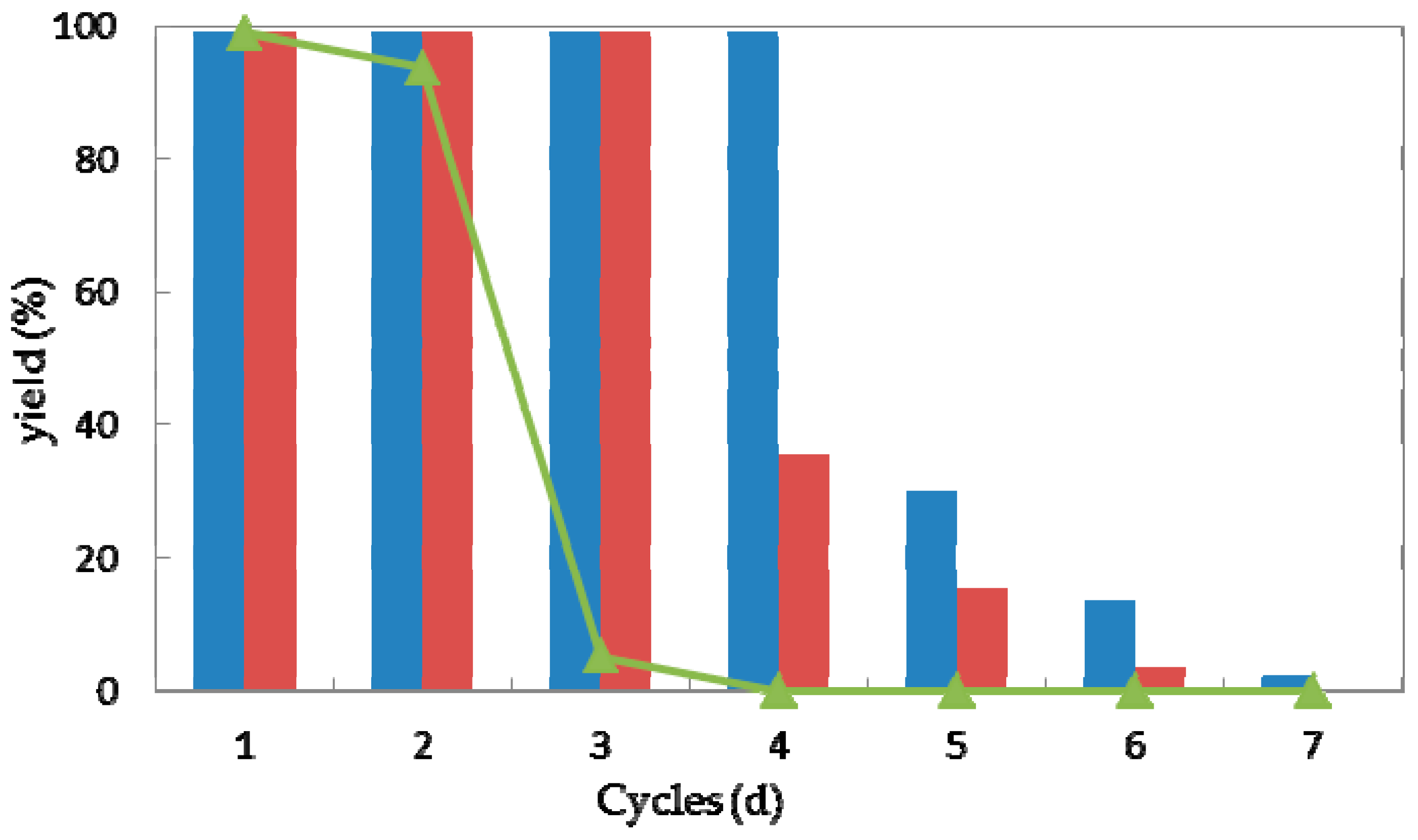
2.3.4. Reusability
3. Materials and Methods
3.1. General
3.2. Immobilization of G. candidum AS 2.361
3.2.1. Agar Immobilization
3.2.2. Calcium Alginate Immobilization
3.3. SEM Analysis
3.4. Reduction of Ketones
3.5. Preparative-Scale Synthesis of (S)-1-(2-bromophenyl) ethan-1-ol (S-2a)
3.6. pH Tolerance and Thermostability
3.7. Storage Stability and Reusability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, J.H.; Xu, Y. Isolation of a bacillus strain producing ketone reductase with high substrate tolerance. Bioresour. Technol. 2010, 101, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chandrani, M.; Hua, L. ‘Green’ synthesis of important pharmaceutical building blocks: Enzymatic access to enantiomerically pure α-chloroalcohols. Tetrahedron Asymmetry 2005, 16, 3275–3278. [Google Scholar] [CrossRef]
- Hiraishi, T.; Taguchi, S. Enzyme-catalyzed synthesis and degradation of biopolymers. Org. Chem. 2009, 6, 44–54. [Google Scholar] [CrossRef]
- Faber, K. Biotransformations in Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–30. [Google Scholar]
- Zheng, Y.G.; Yin, H.H.; Yu, D.F.; Chen, X.; Tang, X.L.; Zhang, X.J.; Xue, Y.P.; Wang, Y.J.; Liu, Z.Q. Recent advances in biotechnological applications of alcohol dehydrogenases. Appl. Microbiol. Biol. 2017, 101, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zong, M.H.; Lou, W.Y. Use of an ionic liquid to improve asymmetric reduction of 4′-methoxyacetophenone catalyzed by immobilized Rhodotorula sp. AS2. 2241 cells. J. Mol. Catal. B Enzym. 2009, 56, 70–76. [Google Scholar] [CrossRef]
- Kollerov, V.V.; Shutov, A.A.; Fokina, V.V.; Sukhodol’skaya, G.V.; Donova, M.V. Biotransformation of 3-keto-androstanes by Gongronella butleri VKM F-1033. J. Mol. Catal. B Enzym. 2008, 55, 61–68. [Google Scholar] [CrossRef]
- Carballeira, J.; Quezada, M.; Hoyos, P.; Simeó, Y.; Hernaiz, M.; Alcantara, A.; Sinisterra, J. Microbial cells as catalysts for stereoselective red–ox reactions. Biotechnol. Adv. 2009, 27, 686–714. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Xiao, R.; Xu, Y.; Montelione, G.T. Novel anti-Prelog stereospecific carbonyl reductases from Candida parapsilosis for asymmetric reduction of prochiral ketones. Org. Biomol. Chem. 2011, 9, 4070–4078. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.C.; Ferreira, H.V.; Pimenta, E.F.; Berlinck, R.G.S.; Rezende, M.O.O.; Landgraf, M.D.; Seleghim, M.H.R.; Sette, L.D.; Porto, A.L.M. Biotransformation of α-bromoacetophenones by the marine fungus Aspergillus sydowii. Mar. Biotechnol. 2010, 12, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.C.; Ferreira, H.V.; Luiz, R.F.; Sette, L.D.; Porto, A.L.M. Stereoselective bioreduction of 1-(4-methoxyphenyl) ethanone by whole cells of marine-derived fungi. Mar. Biotechnol. 2012, 14, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.C.; Seleghim, M.H.R.; Comasseto, J.V.; Sette, L.D.; Porto, A.L.M. Stereoselective bioreduction of α-azido ketones by whole cells of marine-derived fungi. Mar. Biotechnol. 2015, 17, 736–742. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; Seleghim, M.H.R.; Porto, A.L.M. Biotransformation of methylphenylacetonitriles by Brazilian marine fungal strain Aspergillus sydowii CBMAI 934: eco-friendly reactions. Mar. Biotechnol. 2014, 16, 156–160. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, V.; Guidi, B.; Contente, M.L.; Granato, T.; Conti, P.; Molinari, F.; Crotti, E.; Mapelli, F.; Borin, S.; Daffonchio, D.; et al. Marine microorganisms as source of stereoselective esterases and ketoreductases: kinetic resolution of a prostaglandin intermediate. Mar. Biotechnol. 2015, 17, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Pramanik, A.; Mitra, A.; Mukherjee, J. Bioprocessing data for the production of marine enzymes. Mar. Drugs 2010, 8, 1323–1372. [Google Scholar] [CrossRef] [PubMed]
- Trincone, A. Potential biocatalysts originating from sea environments. J. Mol. Catal. B Enzym. 2010, 66, 241–256. [Google Scholar] [CrossRef]
- Trincone, A. Marine biocatalysts: enzymatic features and applications. Mar. Drugs 2011, 9, 478–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; de Souza, F.Z.R.; Liu, L.; Chen, B.S. The use of marine-derived fungi for preparation of enantiomerically pure alcohols. Appl. Microbiol. Biol. 2018, 102, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, B.S.; de Souza, F.Z.R.; Liu, L. A comparative study on asymmetric reduction of ketones using the growing and resting cells of marine-derived fungi. Mar. Drugs 2018, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Duan, W.-D.; de Souza, F.Z.R.; Liu, L.; Chen, B.-S. Asymmetric ketone reduction by immobilized Rhodotorula mucilaginosa. Catalysts 2018, 8, 165. [Google Scholar] [CrossRef]
- Azerad, R.; Buisson, D. Microbial Reagents in Organic Synthesis; Servi, S., Ed.; Kluwer Academic: Dordrech, The Netherlands, 2017; pp. 1–30. [Google Scholar]
- Nakamura, K.; Matsuda, T. Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum. J. Org. Chem. 1998, 63, 8957–8964. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitano, K.; Matsuda, T.; Ohno, A. Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum. Tetrahedron Lett. 1996, 10, 1629–1632. [Google Scholar] [CrossRef]
- Rao, N.N.; Lütz, S.; Seelbach, K.; Liese, A. Basics of bioreaction engineering. In Industrial Biotransformations, 2nd ed.; Liese, A., Seelbach, K., Wandrey, C., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 115–145. [Google Scholar]
- Schrewe, M.; Julsing, M.K.; Bhler, B.; Schmid, A. Whole-cell biocatalysis for selective and productive C–O functional group introduction and modification. Chem. Soc. Rev. 2013, 42, 6346–6377. [Google Scholar] [CrossRef] [PubMed]
- Quezada, M.A.; Carbaleira, J.D.; García-Burgos, C.A.; Sinisterra, J.V. Monascus kaoliang CBS 302.78 immobilized in tailor-made agars as catalyst for reduction of ketones: n the quest for a green biocatalyst. Process Biochem. 2008, 43, 1220–1226. [Google Scholar] [CrossRef]
- Melo, A.D.Q.; Silva, F.F.M.; dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Filho, F.A.D. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Huang, K.L.; Jiang, Y.R.; Ding, P. Production of (R)-mandelic acid by immobilized cells of Saccharomyces cerevisiae on chitosan carrier. Process Biochem. 2007, 42, 1465–1469. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zheng, W.; Meng, Z.X.; Zhou, H.M.; Xu, X.X.; Li, Z.; Zheng, Y.F. Bioelectrochemistry of hemoglobin immobilized on a sodium alginate-multiwall carbon nanotubes composite film. Biosens. Bioelectron. 2009, 24, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Suzana, W. Effect of sodium alginate concentration, bead diameter, initial pH and temperature on lactic acid production from pineapple waste using immobilized Lactobacillus delbrueckii. Process Biochem. 2006, 41, 1117–1123. [Google Scholar] [CrossRef]
- Rocha, L.C.; de Souza, A.L.; Filho, U.P.R.; Filho, S.P.C.; Sette, L.D.; Porto, A.L.M. Immobilization of marine fungi on silica gel, silica xerogel and chitosan for biocatalytic reduction of ketones. J. Mol. Catal. B Enzym. 2012, 84, 160–165. [Google Scholar] [CrossRef]
- Kantam, M.L.; Laha, S.; Yadav, J.; Likhar, P.R.; Sreedhar, B.; Jha, S.; Bhargava, S.; Udayakiran, M.; Jagadeesh, B. An efficient copper-aluminum hydrotalcite catalyst for asymmetric hydrosilylation of ketones at room temperature. Org. Lett. 2008, 10, 2979–2982. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Harada, T. Asymmetric mukaiyama aldol reaction of nonactivated ketones catalyzed by allo-threonine-derived oxazaborolidinone. Org. Lett. 2008, 10, 4999–5001. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.E.D.; Morris, D.J.; Wills, M. Asymmetric hydrogenation of ketones using Ir(III) complexes of N-alkyl-N’-tosyl-1,2-ethanediamine ligands. Tetrahedron Lett. 2009, 50, 688–692. [Google Scholar] [CrossRef]
- Gilmore, N.J.; Jones, S.; Muldowney, M.P. Synthetic applicability and in situ recycling of a β-methoxy oxazaborolidine catalyst derived from cis-1-amino-indan-2-ol. Org. Lett. 2004, 6, 2805–2808. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.P.; Vila-Real, H.; Fernandes, P.C.B.; Ribeiro, M.H.L. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Biochem. Biotechnol. 2010, 160, 2129–2147. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Zain, N.A.M.; Suhaimi, M.S. Immobilization of Baker’s yeast invertase in PVA–alginate matrix using innovative immobilization technique. Process Biochem. 2008, 43, 331–338. [Google Scholar] [CrossRef]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.-Y.; Juang, R.-S. Use of chitosan-clay composite as immobilization support for improved activity and stability of β-glucosidase. Biochem. Eng. J. 2007, 35, 93–98. [Google Scholar] [CrossRef]
- Poyatos, M.; Marquez, F.; Peris, E.; Claverb, C.; Fernandez, E. Preparation of a new clay-immobilized highly stable palladium catalyst and its efficient recyclability in the Heck reaction. New J. Chem. 2003, 27, 425–431. [Google Scholar] [CrossRef]
- Castro, H.F.; Silva, M.L.C.P.; Silva, G.L.J.P. Evaluation of inorganic matrixes as supports for immobilization of microbial lipase. Braz. J. Chem. Eng. 2008, 17, 4–7. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Villalb, M.; Verdasco-Martín, C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R.; Otero, C. Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzyme Microb. Technol. 2016, 90, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Cruz, J.; Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Villalonga, R.; Fernandez-Lafuente, R. Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv. 2016, 6, 27329–27334. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1a-1j, (S)-2a, (S)-2b, (S)-2c, (S)-2d, (S)-2e, (S)-2f, (S)-2g, (S)-2h, (S)-2i, (R)-2i, (S)-2j are available from the authors. |







| Substrate | Free Cells | Immobilized Cells on Agar | Immobilized Cells on Calcium Alginate | |||
|---|---|---|---|---|---|---|
| Yield (%) | ee (%) | Yield (%) | ee (%) | Yield (%) | ee (%) | |
| 1a | 99 | 99 (S) | 99 | 99 (S) | 99 | 99 (S) |
| 1b | 44 | 58 (S) | 65 | 99 (S) | 39 | 74 (S) |
| 1c | 49 | 75 (S) | 37 | 8 (S) | 45 | 32 (S) |
| 1d | 81 | 90 (S) | 96.7 | 99 (S) | 33 | 99 (S) |
| 1e | 96 | 99 (S) | 66 | 99 (S) | 89 | 91 (S) |
| 1f | 54 | 59 (S) | 53 | 32 (S) | 35 | 19 (S) |
| 1g | 63 | 87 (S) | 86 | 99 (S) | 28 | 99 (S) |
| 1h | 52 | 81 (S) | 36 | 97 (S) | 48 | 99 (S) |
| 1i | 24 | 18 (S) | 41.5 | 45 (R) | 39 | 38 (R) |
| 1j | 41 | 87 (R) | 34.3 | 99 (R) | 33 | 69 (R) |
| 1k | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1l | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1m | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1n | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; De Souza, F.Z.R.; Liu, L.; Chen, B.-S. Immobilized and Free Cells of Geotrichum candidum for Asymmetric Reduction of Ketones: Stability and Recyclability. Molecules 2018, 23, 2144. https://doi.org/10.3390/molecules23092144
Liu H, De Souza FZR, Liu L, Chen B-S. Immobilized and Free Cells of Geotrichum candidum for Asymmetric Reduction of Ketones: Stability and Recyclability. Molecules. 2018; 23(9):2144. https://doi.org/10.3390/molecules23092144
Chicago/Turabian StyleLiu, Hui, Fayene Zeferino Ribeiro De Souza, Lan Liu, and Bi-Shuang Chen. 2018. "Immobilized and Free Cells of Geotrichum candidum for Asymmetric Reduction of Ketones: Stability and Recyclability" Molecules 23, no. 9: 2144. https://doi.org/10.3390/molecules23092144






