Natural Scaffolds with Multi-Target Activity for the Potential Treatment of Alzheimer’s Disease
Abstract
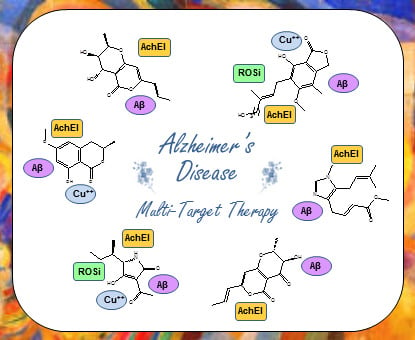
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Production, Isolation, and Identification of Radicinin
3.2. Inhibition of Aβ1–40 Aggregation
3.3. AChE and BChE Inhibition
3.4. Antioxidant Activity (DPPH Method)
3.5. Metal-Ligands Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Have there been improvements in Alzheimer’s disease drug discovery over the past 5 years? Expert Opin. Drug Discov. 2018, 13, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Wang, W.; Wu, J.J.; Liu, L.; Theodoratou, E.; Car, J.; Middleton, L.; Russ, T.C.; Deary, I.J.; Campbell, H.; et al. Global Health Epidemiology Reference Group (GHERG). Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet 2013, 381, 2016–2023. [Google Scholar] [CrossRef]
- Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Hogan, D.B.; Smith, E.E.; Frolkis, A.; Cohen, A.; Kirk, A.; Pearson, D.; Pringsheim, T.; et al. The prevalence and incidence of dementia due to Alzheimer’s disease: A systematic review and meta-analysis. Can. J. Neurol. Sci. 2016, 43, S51–S82. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Neurological Disorders Collaborator Group. 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frölich, L.; Jack, C.R., Jr.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Senol, F.S. Designing Multi-Targeted Therapeutics for the Treatment of Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Daoud, I.; Melkemi, N.; Salah, T.; Ghalem, S. Combined QSAR, molecular docking and molecular dynamics study on new Acetylcholinesterase and Butyrylcholinesterase inhibitors. Comput. Biol. Chem. 2018, 74, 304–326. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L. New approaches for prevention and treatment of Alzheimer’s disease: A fascinating challenge. Neural Regen. Res. 2017, 12, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.; Capone, R.; Cauvi, D.M.; Arispe, N.; De Maio, A. Modulation of Alzheimer’s amyloid β peptide oligomerization and toxicity by extracellular Hsp70. Cell Stress Chaperones 2018, 23, 269–279. [Google Scholar] [CrossRef]
- Chaves, S.; Piemontese, L.; Hiremathad, A.; Santos, M.A. Hydroxypyridinone derivatives: A fascinating class of chelators with therapeutic applications—An update. Curr. Med. Chem. 2018, 25, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in multifunctional metal chelators as potential drugs for Alzheimer’s disease. Coord. Chem. Rev. 2016, 327–328, 287–303. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Telpoukhovskaia, M.; Orvig, C. The art of building multifunctional metal-binding agents from basic molecular scaffolds for the potential application in neurodegenerative diseases. Coord. Chem. Rev. 2012, 256, 2308–2332. [Google Scholar] [CrossRef]
- Savelieff, M.G.; DeToma, A.S.; Derrick, J.S.; Lim, M.H. The ongoing search for small molecules to study metal associated amyloidβ species in Alzheimer’s disease. Acc. Chem. Res. 2014, 47, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L.; Fracchiolla, G.; Carrieri, A.; Parente, M.; Laghezza, A.; Carbonara, G.; Sblano, S.; Tauro, M.; Gilardi, F.; Tortorella, P.; et al. Design, synthesis and biological evaluation of a class of bioisosteric oximes of the novel dual peroxisome proliferator-activated receptor α/γ ligand LT175. Eur. J. Med. Chem. 2015, 90, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Fracchiolla, G.; Laghezza, A.; Piemontese, L.; Parente, M.; Lavecchia, A.; Pochetti, G.; Montanari, R.; Di Giovanni, C.; Carbonara, G.; Tortorella, P.; et al. Synthesis, Biological Evaluation and Molecular Investigation of Fluorinated PPARalpha/gamma Dual Agonists. Bioorg. Med. Chem. 2012, 20, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L. Plant Food Supplements with Antioxidant Properties for the Treatment of Chronic and Neurodegenerative Diseases: Benefits or Risks? J. Diet. Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A. A review: Natural compounds as anti-Alzheimer’s Disease agents. Curr. Food Nutr. Sci. 2017, 13, 247–254. [Google Scholar] [CrossRef]
- Anand, P.; Singh, P.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Visconti, A. Anticholinesterase activity of the Fusarium metabolite visoltricin and its N-methyl derivative. Toxicology 1994, 8, 461–465. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Vitti, C.; De Girolamo, A.; Visconti, A.; Logrieco, A.; Fanizzi, F.P. Radicinols and Radicinin Phytotoxins Produced by Alternaria radicina on Carrots. J. Agric. Food Chem. 2004, 52, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; De Girolamo, A.; Vitti, C.; Tylkowska, K.; Grabarkiewicz-Szczęsna, J.; Szopińska, D.; Dorna, H. Toxigenic profile of Alternaria alternata and Alternaria radicina occurring on umbelliferous plants. Food Addit. Contam. 2005, 22, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.M. Influence of the new immunosuppressive combinations on arterial hypertension after renal transplantation. Kidney Intern. 2002, 62, S81–S87. [Google Scholar] [CrossRef] [PubMed]
- Epinette, W.W.; Parker, C.M.; Jones, E.L.; Greist, M.C. Mycophenolic acid for psoriasis. A review of pharmacology, long-term efficacy, and safety. J. Am. Acad. Dermatol. 1987, 17, 962–971. [Google Scholar] [CrossRef]
- De Girolamo, A.; Solfrizzo, M.; Vitti, C.; Visconti, A. Occurrence of 6-Methoxymellein in Fresh and Processed Carrots and Relevant Effect of Storage and Processing. J. Agric. Food Chem. 2004, 52, 6478–6484. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Heale, J.B. Cell death, 6-methoxymellein accumulation, and induced resistance to Botrytis cinerea in carrot root slices. Physiol. Mol. Plant Pathol. 1987, 30, 67–75. [Google Scholar] [CrossRef]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce’s Disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Solfrizzo, M. 3-/1-Methyl-4-(3-methyl-2-butenyl)-imidazol-5-yl-2-propenylic Acid Methyl Ester and Its Salts, Isolation Process, Pharmaceutical and Insecticidal Compositions Containing It. Italian Patent n. 22630, 6 December 1989. [Google Scholar]
- Visconti, A.; Solfrizzo, M. Isolation, characterization and biological activity of visoltricin, a novel metabolite of Fusarium tricinctum. J. Agric. Food Chem. 1994, 42, 195–199. [Google Scholar] [CrossRef]
- Rieder, J.M.; Lepschy, J. Synthesis of visoltricin and fungerin: Imidazole derivatives of Fusarium sp. Tetrahedron Lett. 2002, 43, 2375–2376. [Google Scholar] [CrossRef]
- Koizumi, Y.; Arai, M.; Tomoda, H.; Omura, S. Fungerin, a fungal alkaloid, arrests the cell cycle in M phase by inhibition of microtubule polymerization. J. Antibiot. 2004, 57, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Visconti, A. Production and isolation of Alternaria alternata mycotoxins. In Proceedings of the Chemio Forum Research 90, Scientific Research Perspectives for Southern Italy, Battipaglia, Italy, 25–30 May 1990; p. 123. [Google Scholar]
- Nukina, M.; Marumo, S. Radicinol, a new metabolite of Cochliobolus lunata, and absolute stereochemistry of radicinin. Tetrahedron Lett. 1977, 37, 3271–3272. [Google Scholar] [CrossRef]
- Olivieri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef] [PubMed]
- Bareggi, S.R.; Braida, D.; Pollera, C.; Bondiolotti, G.; Formentin, E.; Puricelli, M.; Poli, G.; Ponti, W.; Sala, M. Effects of clioquinol on memory impairment and the neurochemical modifications induced by scrapie infection in golden hamsters. Brain Res. 2009, 1280, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Yan, J.; Li, J.; Jia, X.; Miao, H.; Sun, Y.; Huang, L.; Li, X. New multi-target-directed small molecules against Alzheimer’s disease: A combination of resveratrol and clioquinol. Org. Biomol. Chem. 2014, 12, 5936–5944. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; De Palma, A.; Giangregorio, N.; Miniero, D.V.; Pesce, P.; Nicolotti, O.; Campagna, F.; Altomare, C.D.; Catto, M. Mannich base approach to 5-methoxyisatin 3-(4-isopropylphenyl)hydrazone: A water-soluble prodrug for a multitarget inhibition of cholinesterases, beta-amyloid fibrillization and oligomer-induced cytotoxicity. Eur. J. Pharm. Sci. 2017, 109, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Seong, S.H.; Reddy, M.R.; Seo, S.Y.; Choi, J.S.; Jung, H.A. Kinetics and Molecular Docking Studies of 6-Formyl Umbelliferone Isolated from Angelica decursiva as an Inhibitor of Cholinesterase and BACE1. Molecules 2017, 22, 1604. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Kaur, A.; Kaur, R.; Yadav, A.K.; Sharma, V.; Chadha, B.S. Cholinesterase inhibitor (Altenuene) from an endophytic fungus Alternaria alternata: Optimization, purification and characterization. J. Appl. Microbiol. 2016, 121, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Senol, F.S.; Shekfeh, S.; Skalicka-Wozniak, K.; Banoglu, E. Pteryxin—A promising butyrylcholinesterase-inhibiting coumarin derivative from Mutellina purpurea. Food Chem. Toxic. 2017, 109, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Chand, K.; Esteves, A.R.; Cardoso, S.M.; Ramsay, R.R.; Chaves, S.; Keri, R.S.; Santos, M.A. Tacrine-allyl/propargylcysteine-benzothiazole trihybrids as potential anti-Alzheimer’s drug candidates. RSC Adv. 2016, 6, 53519–53532. [Google Scholar] [CrossRef]
- Richard, T.; Pawlus, A.D.; Iglésias, M.-L.; Pedrot, E.; Waffo-Teguo, P.; Mérillon, J.-M.; Monti, J.-P. Neuroprotective properties of resveratrol and derivatives. Ann. N.Y. Acad. Sci. 2011, 1215, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pate, K.M.; Rogers, M.; Reed, J.W.; van der Munnik, N.; Vance, S.Z.; Mossa, M.A. Anthoxanthin polyphenols attenuate Aβ oligomer-induced neuronal responses associated with Alzheimer’s disease. CNS Neurosci. Ther. 2017, 23, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B. Natural antioxidants for neurodegenerative diseases. Mol. Neurobiol. 2005, 31, 283–293. [Google Scholar] [CrossRef]
- Smith, W.W.; Gorospe, M.; Kusiak, J.W. Signaling mechanisms underlying Aβ toxicity: Potential therapeutic targets for Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2006, 5, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Jalili-Baleh, L.; Babaei, E.; Abdpour, S.; Bukhari, S.N.A.; Foroumadi, A.; Ramazani, A.; Sharifzadeh, M.; Abdollahi, M.; Khoobi, M. A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 152, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Bergamini, C.; Fato, R.; Soukup, O.; Korabecny, J.; Andrisano, V.; Bartolini, M.; Bolognesi, M.L. Novel 8-Hydroxyquinolin derivatives as Multitarget compounds for the treatment of Alzheimer’s disease. Chem. Med. Chem. 2016, 11, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9, S3. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feartherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Bruno, C.; Lovece, A.; Roselli, M.G.; Cavalluzzi, M.M.; De Santis, F.; De Palma, A.; Rusciano, M.R.; Illario, M.; et al. N-(Phenoxyalkyl)amides as MT1 and MT2 ligands: Antioxidant properties and inhibition of Ca2+/CaM-dependent kinase II. Bioorg. Med. Chem. 2013, 21, 847–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauro, M.; Laghezza, A.; Loiodice, F.; Piemontese, L.; Caradonna, A.; Capelli, D.; Montanari, R.; Pochetti, G.; Di Pizio, A.; Agamennone, M.; et al. Catechol-based matrix metalloproteinase inhibitors with additional antioxidative activity. J. Enzyme Inhib. Med. Chem. 2016, 31, S25–S37. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| eeAChEi IC50 (μM ± SEM) | esBChEi IC50 (μM ± SEM) | Antioxidant Activity EC50 (μmol/µmol of DPPH ± SEM) | iAβ IC50 (μM ± SEM) | |
|---|---|---|---|---|
| Galantamine | 0.51 ± 0.10 | 8.70 ± 1.02 | n.d. | n.d. |
| Gallic acid | n.d. | n.d. | 0.054 ± 0.004 | n.d. |
| Quercetin | n.d. | n.d. | n.d. | 0.82 ± 0.07 |
| Clioquinol | 8.12 ± 1.00 | %I (10μM): 10 ± 1% | 0.74 ± 0.04 | 7.6 ± 0.8 |
| 1 | 8.13 ± 0.08 | %I (10μM): 7 ± 1% | 2.6 ± 0.2 | %I (100μM): 50 ± 8 |
| 2 | 6.86 ± 0.67 | i.a. | > 100 | 74 ± 1 |
| 3 | 7.84 ± 0.72 | i.a. | 14.7 ± 3.4 | %I (100μM): 38 ± 3 |
| 4 | 11.4 ± 0.8 | %I (10μM): 10 ± 3% | > 100 | 98 ± 3 |
| 5 | 8.96 ± 0.97 | %I (10μM): 6 ± 1% | >100 | %I (100μM): 44 ± 3 |
| 6 | 86.0 ± 15.0 | 1.75 ± 0.59 | >100 | %I (100μM): 33 ± 9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piemontese, L.; Vitucci, G.; Catto, M.; Laghezza, A.; Perna, F.M.; Rullo, M.; Loiodice, F.; Capriati, V.; Solfrizzo, M. Natural Scaffolds with Multi-Target Activity for the Potential Treatment of Alzheimer’s Disease. Molecules 2018, 23, 2182. https://doi.org/10.3390/molecules23092182
Piemontese L, Vitucci G, Catto M, Laghezza A, Perna FM, Rullo M, Loiodice F, Capriati V, Solfrizzo M. Natural Scaffolds with Multi-Target Activity for the Potential Treatment of Alzheimer’s Disease. Molecules. 2018; 23(9):2182. https://doi.org/10.3390/molecules23092182
Chicago/Turabian StylePiemontese, Luca, Gabriele Vitucci, Marco Catto, Antonio Laghezza, Filippo Maria Perna, Mariagrazia Rullo, Fulvio Loiodice, Vito Capriati, and Michele Solfrizzo. 2018. "Natural Scaffolds with Multi-Target Activity for the Potential Treatment of Alzheimer’s Disease" Molecules 23, no. 9: 2182. https://doi.org/10.3390/molecules23092182
APA StylePiemontese, L., Vitucci, G., Catto, M., Laghezza, A., Perna, F. M., Rullo, M., Loiodice, F., Capriati, V., & Solfrizzo, M. (2018). Natural Scaffolds with Multi-Target Activity for the Potential Treatment of Alzheimer’s Disease. Molecules, 23(9), 2182. https://doi.org/10.3390/molecules23092182