Development and Evaluation of Minocycline Hydrochloride-Loaded In Situ Cubic Liquid Crystal for Intra-Periodontal Pocket Administration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of Precursor Formulations
2.2. Physicochemical Characterization
2.3. In Vitro Drug Release Studies
2.4. Evaluation of Phase Behavior
2.5. In Vivo Pharmacodynamics Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of Precursor Formulations
3.3. Evaluation of Phase Behaviour
3.3.1. PLM
3.3.2. SAXS Measurements
3.3.3. Rheological Measurements
3.4. Physicochemical Characterization
3.4.1. Evaluation of Syringeability and Determination of pH Value
3.4.2. Determination of the Vmin
3.4.3. Determination of the Tg
3.5. In Vitro Drug Release
3.6. In Vivo Pharmacodynamics Studies
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000 2016, 71, 82–112. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.S.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000 2015, 67, 131–186. [Google Scholar] [CrossRef] [PubMed]
- Barca, E.; Cifcibasi, E.; Cintan, S. Adjunctive use of antibiotics in periodontal therapy. J. Istanb. Univ. Fac. Dent. 2015, 49, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.C.W.; Dalbó, S.; Striechen, T.M.; Farias, J.M.; Olchanheski, L.R.; Mendes, R.T.; Vellosa, J.C.R.; Fávero, G.M.; Sordi, R.; Assreuy, J.; et al. Experimental periodontitis promotes transient vascular inflammation and endothelial dysfunction. Arch. Oral Biol. 2013, 58, 1187–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, J.D.; Moss, K.L.; Morelli, T. Periodontal profile class is associated with prevalent diabetes, coronary heart disease, stroke, and systemic markers of C-reactive protein and interleukin-6. J. Periodontol. 2018, 89, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; White, S.; Bartold, M. Periodontal disease as a risk factor for rheumatoid arthritis: A systematic review. JBI Libr. Syst. Rev. 2012, 10, 615–678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, W.; Liu, X.; Zhang, W.; Li, Y. Interrelationship between diabetes and periodontitis: Role of hyperlipidemia. Arch. Oral Biol. 2015, 60, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Jhinger, N.; Kapoor, D.; Jain, R. Comparison of periochip (chlorhexidine gluconate 2.5 mg) and arestin (minocycline hydrochloride 1 mg) in the management of chronic periodontitis. Indian. J. Dent. 2015, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. localized in situ nanoemulgel drug delivery system of quercetin for periodontitis: Development and computational simulations. Molecules 2018, 23, 1363. [Google Scholar] [CrossRef] [PubMed]
- Da, R.H.; Silva, C.F.; Santiago, F.L.; Martins, L.G.; Dias, P.C.; De, M.D. Local drug delivery systems in the treatment of periodontitis: A literature review. J. Int. Acad. Periodontol. 2015, 17, 82–90. [Google Scholar]
- Abbas, S.; Mahendra, J.; Ari, G. Minocycline ointment as a local drug delivery in the treatment of generalized chronic periodontitis—A clinical study. J. Clin. Diagn. Res. 2016, 10, 15–19. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.F.; Jorge, A.O.; Dos Santos, S.S. In vitro minocycline activity on superinfecting microorganisms isolated from chronic periodontitis patients. Braz. Oral Res. 2006, 20, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Oringer, R.J.; Shammari, K.F.; Aldredge, W.A.; Iacono, V.J.; Eber, R.M.; Wang, H.L.; Berwald, B.; Nejat, R.; Giannobile, W.V. Effect of locally delivered monicycline microspheres on markers of bone resorption. J. Periodontol. 2002, 73, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Panwar, M.; Gupta, S.H. Local drug delivery with tetracycline fiber: An alternative to surgical periodontal therapy. Med. J. Armed Forces India 2009, 65, 244–246. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Cholesterol in situ, forming gel loaded with doxycycline hyclate for intra-periodontal pocket delivery. Eur. J. Pharm. Sci. 2017, 99, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Schkarpetkin, D.; Reise, M.; Wyrwa, R.; Völpel, A.; Berg, A.; Schweder, M.; Watts, D.C.; Sigusch, B.W. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent. Mater. 2016, 32, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Chanyaboonsub, N.; Setthajindalert, O. Doxycycline hyclate-loaded bleached shellac in situforming microparticle for intraperiodontal pocket local delivery. Eur. J. Pharm. Sci. 2016, 93, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, P.; Pang, Z.; Zhao, J.; Chai, Z.; Li, X.; Li, H.; Jiang, M.; Cheng, H.; Zhang, B.; et al. Local delivery of minocycline-loaded PEG-PLA nanoparticles for the enhanced treatment of periodontitis in dogs. Int. J. Nanomed. 2014, 9, 3963–3970. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. Clinical picture of combination therapy (metronidazole sustained release film with minocycine hydrochloride) in periodontitis. Pak. J. Pharm. Sci. 2015, 28, 397–400. [Google Scholar] [PubMed]
- Agossa, K.; Lizambard, M.; Rongthong, T.; Delcourtdebruyne, E.; Siepmann, J.; Siepmann, F. Physical key properties of antibiotic-free, PLGA/HPMC-based in-situ forming implants for local periodontitis treatment. Int. J. Pharm. 2017, 521, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, S.; Kathpalia, H. Solvent removal precipitation based in situ, forming implant for controlled drug delivery in periodontitis. J. Control. Release 2017, 251, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.D.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and dispersed aqueous phase behavior of phytantriol: Effect of vitamin e acetate and F127 polymer on liquid crystal nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Swarnakar, N.K.; Thanki, K.; Jain, S. Bicontinuous cubic liquid crystalline nanoparticles for oral delivery of doxorubicin: Implications on bioavailability, therapeutic efficacy, and cardiotoxicity. Pharm. Res. 2014, 31, 1219–1238. [Google Scholar] [CrossRef] [PubMed]
- McLain, V.C. Final report on the safety assessment of phytantriol. Int. J. Toxicol. 2007, 26, 107–114. [Google Scholar]
- Chang, C.M.; Bodmeier, R. Low viscosity monoglyceride-based drug delivery systems transforming into a highly viscous cubic phase. Int. J. Pharm. 1998, 173, 51–60. [Google Scholar] [CrossRef]
- Bruschi, M.L.; De, F.O.; Lara, E.H.; Panzeri, H.; Gremião, M.P.; Jones, D.S. Precursor system of liquid crystalline phase containing propolis microparticles for the treatment of periodontal disease: Development and characterization. Drug Dev. Ind. Pharm. 2008, 34, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xie, Y.; Huang, X.; Chen, J.; Huang, Y.; Wang, B.; Wang, H.; Pan, X.; Wu, C. An injectable in situ gel with cubic and hexagonal nanostructures for local treatment of chronic periodontitis. Drug Deliv. 2017, 24, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, K.; Guo, T.; Li, Y.; Zhu, C.; Feng, N. Transdermal baicalin delivery using diethylene glycol monoethyl ether-mediated cubic phase gel. Int. J. Pharm. 2015, 479, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Jasti, B.R. Parenteral routes of delivery. In Theory and Practice of Contemporary Pharmaceutics, 1st ed.; Malanga, C., Rojanasakul, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 387–419. ISBN 9780415288637. [Google Scholar]
- Lara, M.G.; Bentley, M.V.; Collett, J.H. In vitro drug release mechanism and drug loading studies of cubic phase gels. Int. J. Pharm. 2005, 293, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Phelps, J.; Bentley, M.V.; Lopes, L.B. In situ gelling hexagonal phases for sustained release of an anti-addiction drug. Colloid Surf. B 2011, 87, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, X.; Ma, P.; Tao, Y.; Wu, X.; Wu, X.; Chu, X.; Gui, S. Phytantriol-based in situ liquid crystals with long-term release for intra-articular administration. AAPS PharmSciTech 2015, 16, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Mei, L.; Shan, Z.; Huang, Y.; Pan, X.; Li, G.; Gu, Y.; Wu, C. Phytantriol based liquid crystal provide sustained release of anticancer drug as a novel embolic agent. Drug Dev. Ind. Pharm. 2016, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.M.; Davey, G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int. J. Pharm. 2006, 309, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Keyes, P.H.; Jordan, H.V. Periodontal lesions in the syrian hamster findings related to an infectious and transmissible component. Arch. Oral Biol. 1964, 9, 377–379. [Google Scholar] [CrossRef]
- Srivastava, M.; Neupane, Y.R.; Kumar, P.; Kohli, K. Nanoemulgel (NEG) of Ketoprofen with eugenol as oil phase for the treatment of ligature-induced experimental periodontitis in Wistar rats. Drug Deliv. 2015, 23, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Yuanita, T.; Zubaidah, N.; Kunarti, S. Expression of Osteoprotegrin and Osteoclast Level in Chronic Apical Periodontitis Induced with East Java Propolis Extract. Iran. Endod. J. 2018, 13, 42–46. [Google Scholar] [PubMed]
- Yong, C.S.; Choi, J.S.; Quan, Q.Z.; Rhee, J.D.; Kim, C.K.; Lim, S.J.; Kim, K.M.; Oh, P.S.; Choi, H.G. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef]
- Ahmed, A.R.; Dashevsky, A.; Bodmeier, R. Drug release from and sterilization of in situ cubic phase forming monoglyceride drug delivery systems. Eur. J. Pharm. Biopharm. 2010, 75, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Campi, P.; Herrera, B.S.; Jesus, F.N.D.; Napolitano, M.; Teixeira, S.A.; Maia-Dantas, A.; Spolidorio, L.C.; Akamine, E.H.; Mayer, M.P.A.; de Carvalho, M.H.C.; et al. Endothelial dysfunction in rats with ligature-induced periodontitis: Participation of nitric oxide and cycloxygenase-2-derived products. Arch. Oral Biol. 2015, 63, 66–74. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



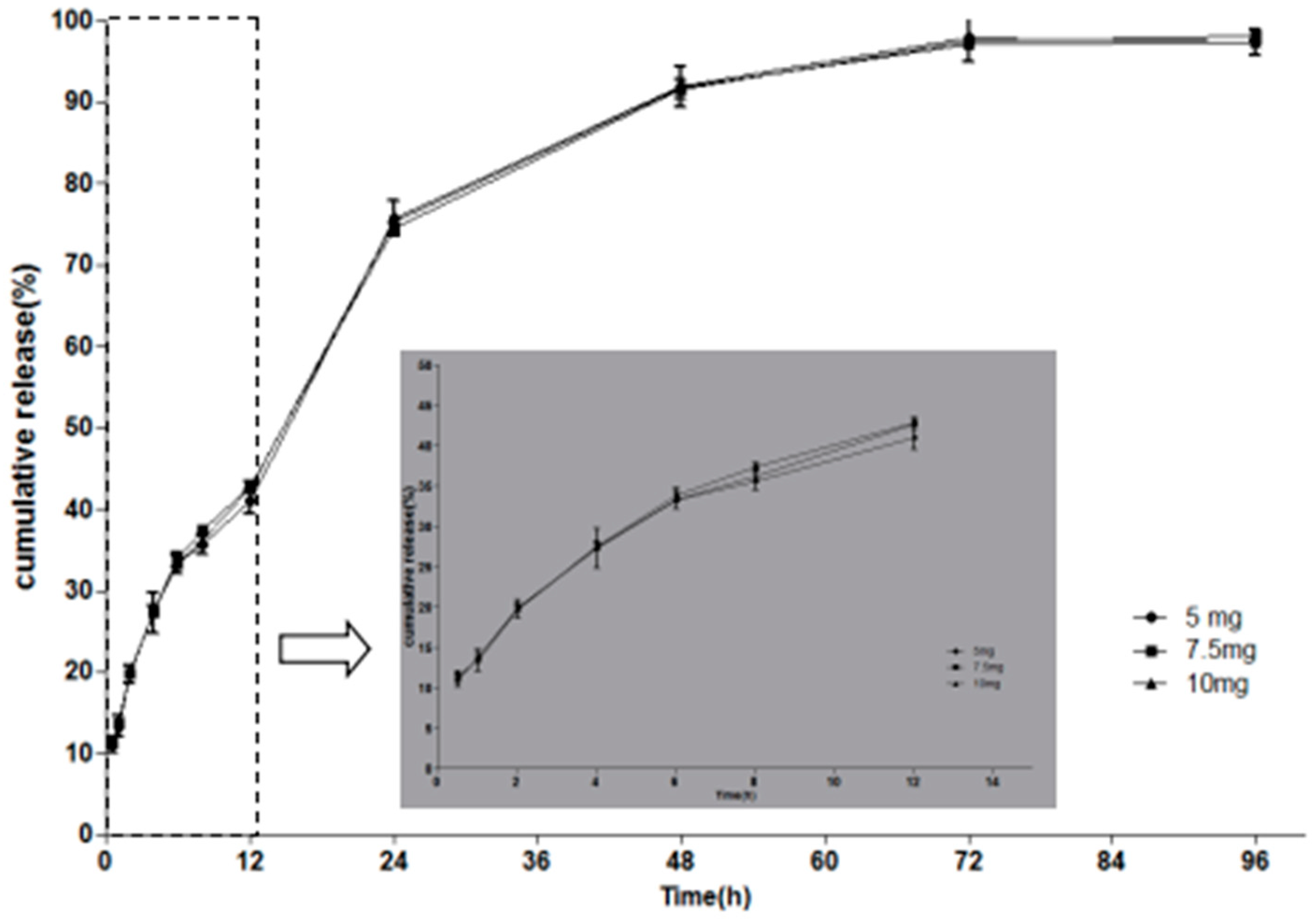





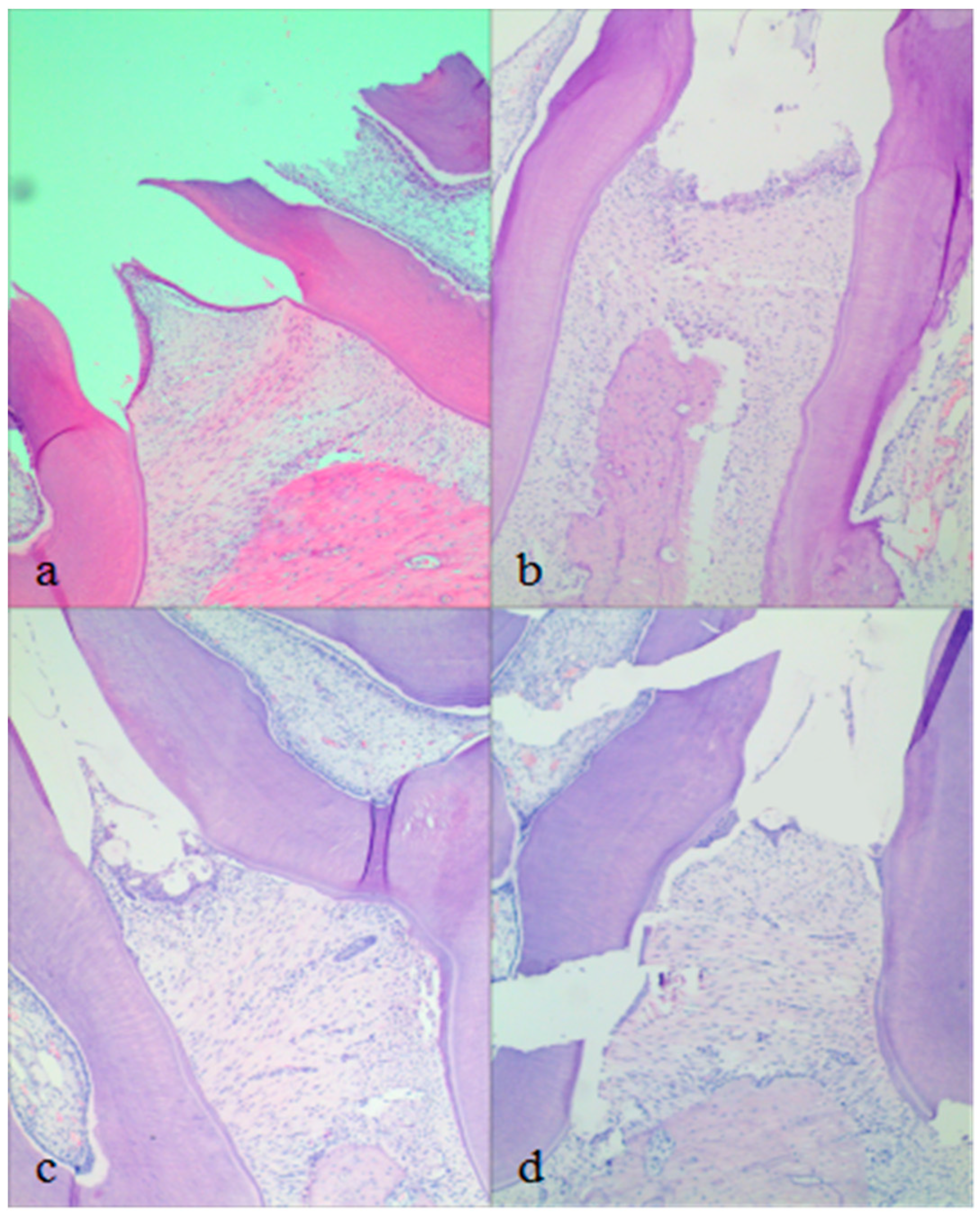
| Formulation | PT (%) | PG (%) | Water (%) | MH (mg/g) |
|---|---|---|---|---|
| F1 | 72 | 18 | 10 | 10 |
| F2 | 63 | 27 | 10 | 10 |
| F3 | 54 | 36 | 10 | 10 |
| F4 | 48 | 32 | 20 | 10 |
| Formulations | Syringeability | pH | Vmin (µL) | Tg (s) |
|---|---|---|---|---|
| F1 | Injectable | 5.22 | 38.12 ± 0.17 | 4.40 ± 0.09 |
| F2 | Injectable | 5.17 | 60.09 ± 0.09 | 6.97 ± 0.10 |
| F3 | Injectable | 5.11 | 74.83 ± 0.22 | 11.70 ± 0.14 |
| F4 | Injectable | 5.25 | 65.5 3 ± 0.12 | 7.09 ± 0.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Liang, X.; Jiang, X.; Guo, J.; Tao, Y.; Wang, S.; Cao, Y.; Gui, S. Development and Evaluation of Minocycline Hydrochloride-Loaded In Situ Cubic Liquid Crystal for Intra-Periodontal Pocket Administration. Molecules 2018, 23, 2275. https://doi.org/10.3390/molecules23092275
Yang Z, Liang X, Jiang X, Guo J, Tao Y, Wang S, Cao Y, Gui S. Development and Evaluation of Minocycline Hydrochloride-Loaded In Situ Cubic Liquid Crystal for Intra-Periodontal Pocket Administration. Molecules. 2018; 23(9):2275. https://doi.org/10.3390/molecules23092275
Chicago/Turabian StyleYang, Zhuanzhuan, Xin Liang, Xiaojing Jiang, Jian Guo, Yaotian Tao, Shengmei Wang, Yingji Cao, and Shuangying Gui. 2018. "Development and Evaluation of Minocycline Hydrochloride-Loaded In Situ Cubic Liquid Crystal for Intra-Periodontal Pocket Administration" Molecules 23, no. 9: 2275. https://doi.org/10.3390/molecules23092275





