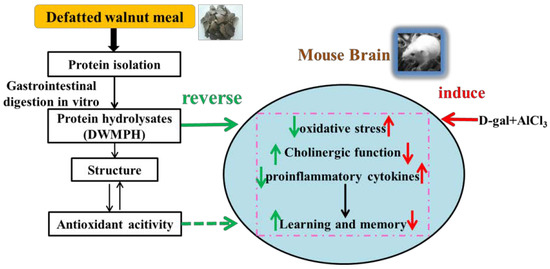
Walnut Protein Hydrolysates Play a Protective Role on Neurotoxicity Induced by d-Galactose and Aluminum Chloride in Mice
Abstract
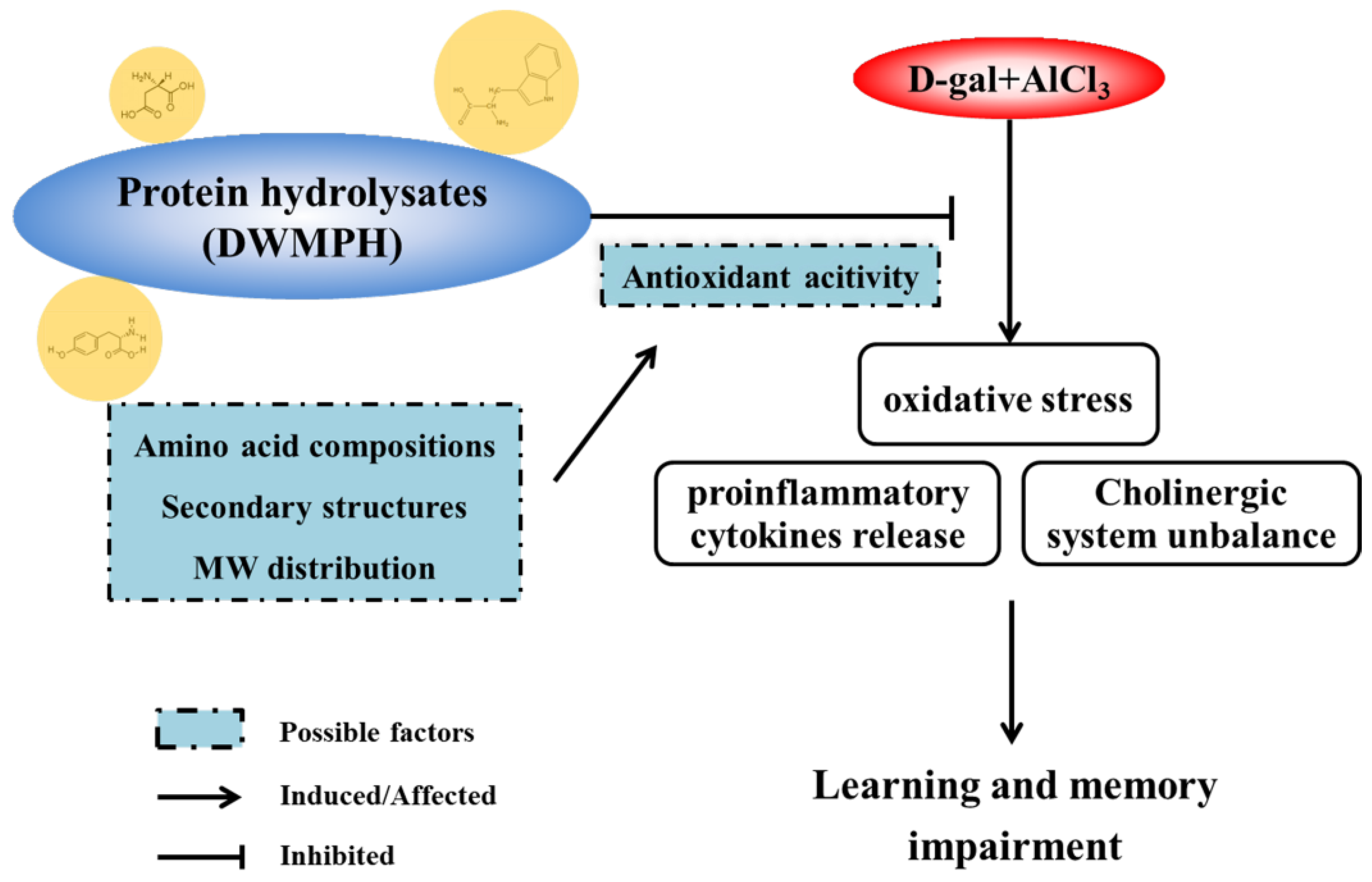
:1. Introduction
2. Results and Discussion
2.1. Body Weight and Food Intake
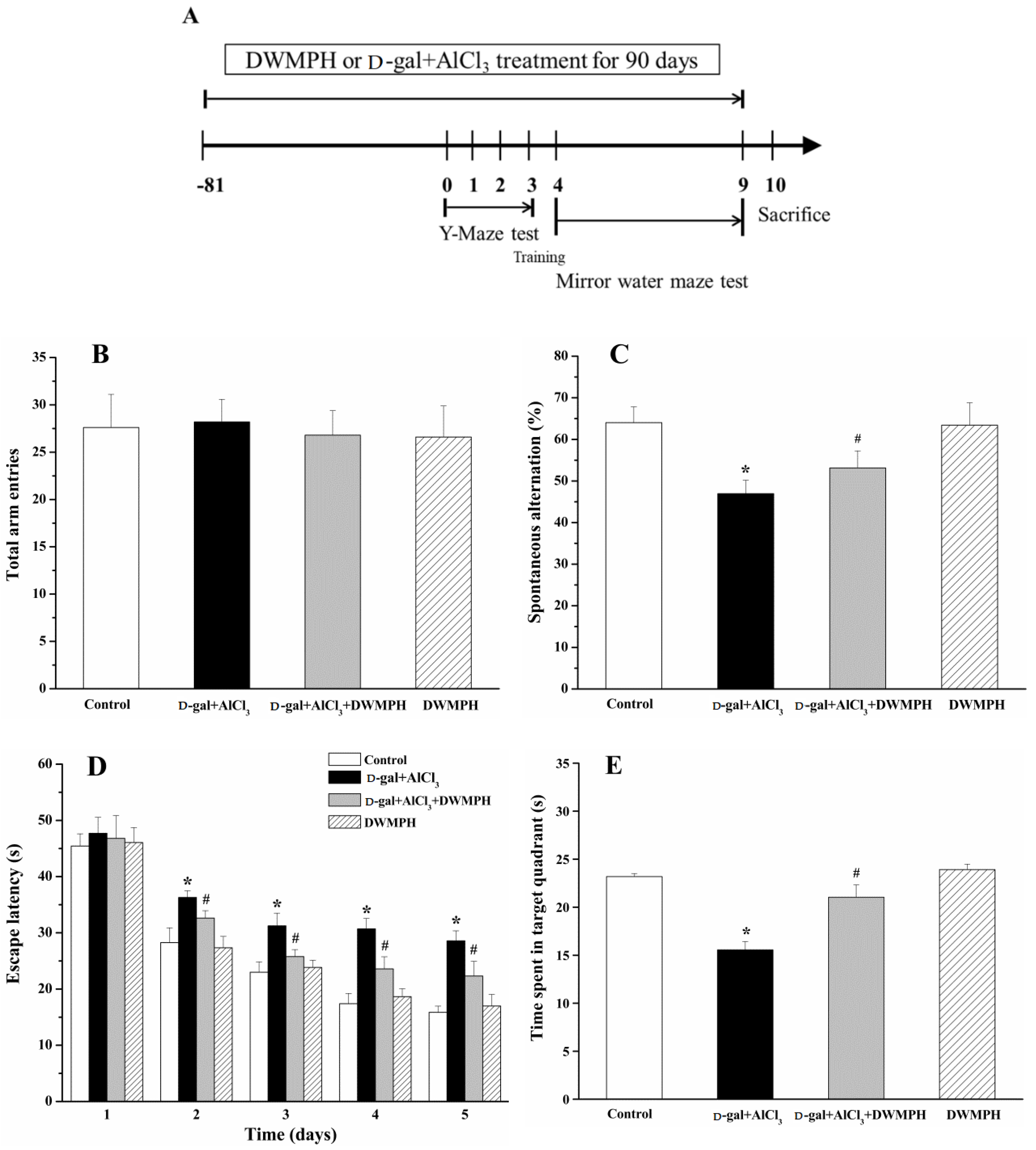
2.2. Effect of DWMPH on Mice Behavioral Test
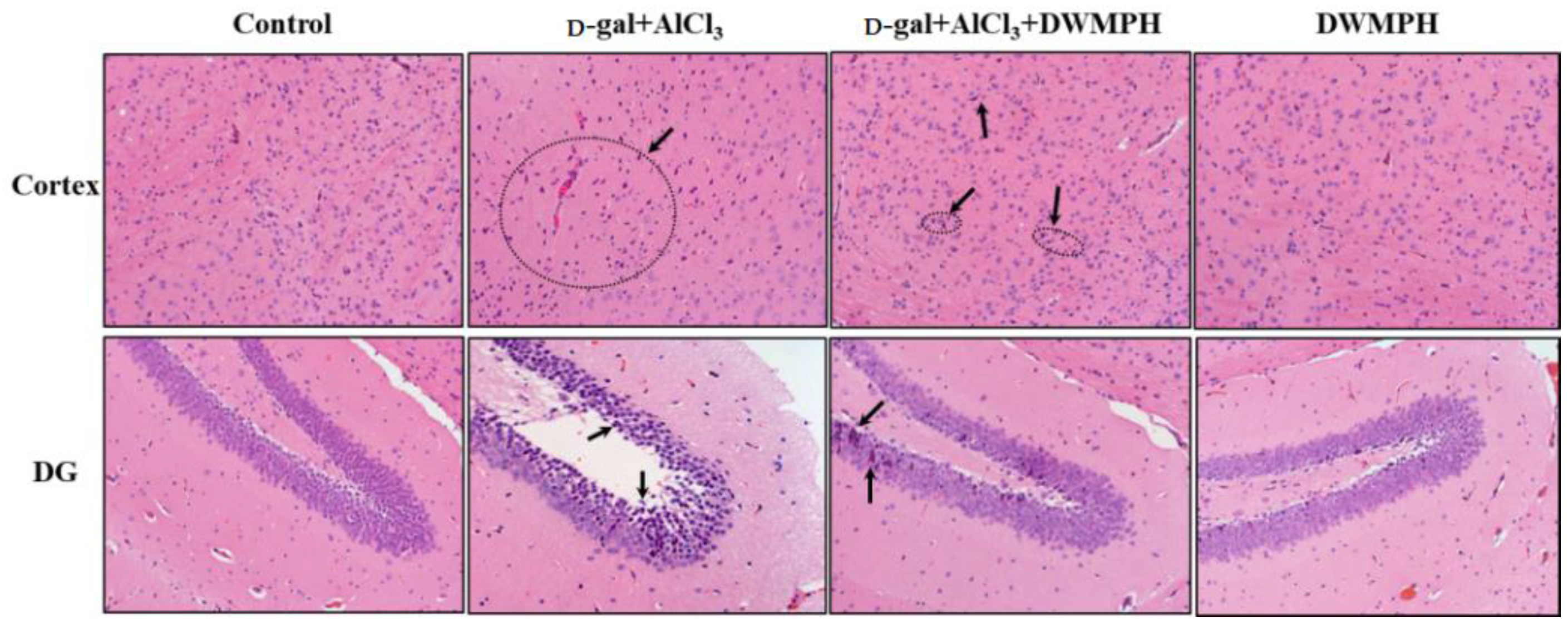
2.3. Effect of DWMPH on Neuronal Morphology
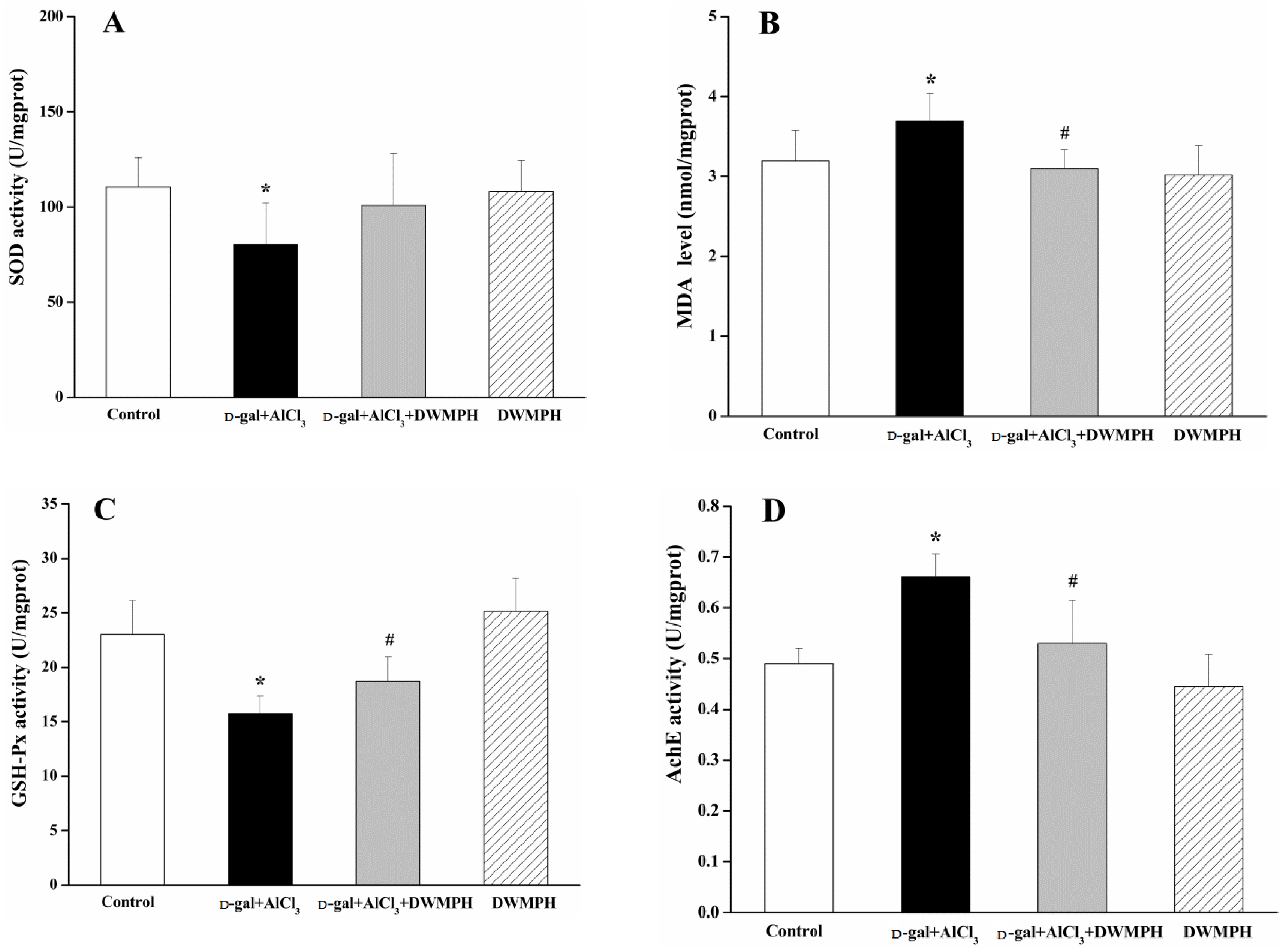
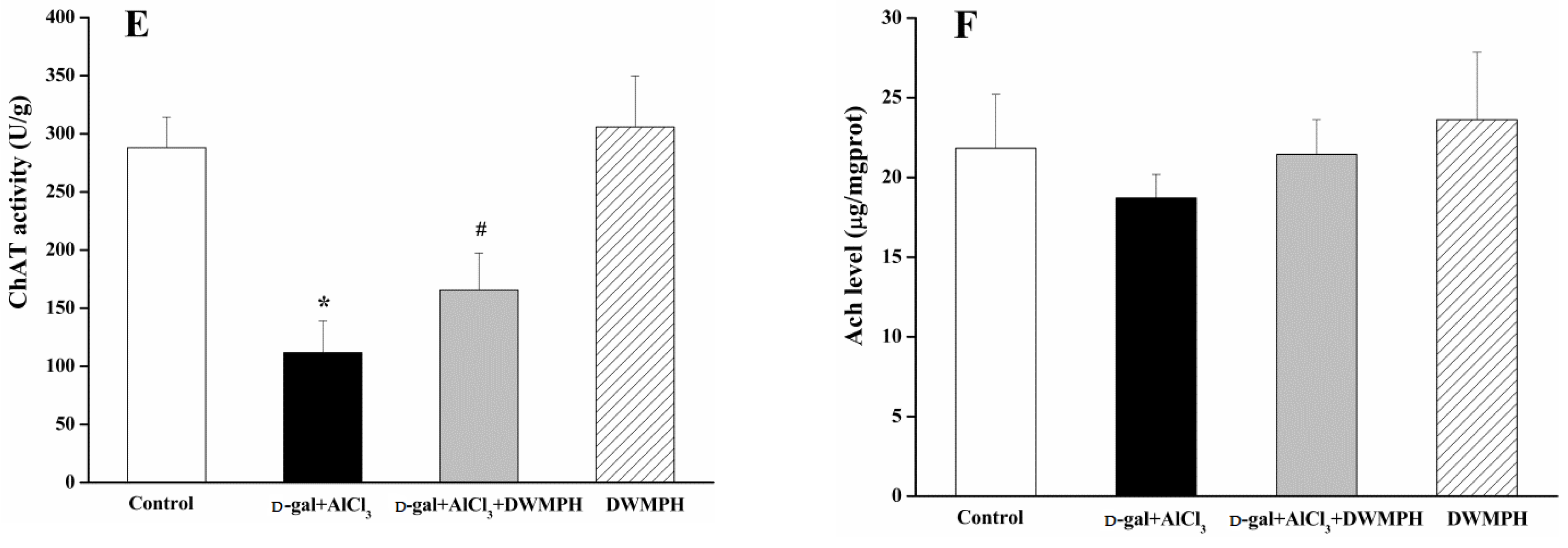
2.4. DWMPH Attenuate Oxidative Damage and Reverse Cholinergic Dysfunction
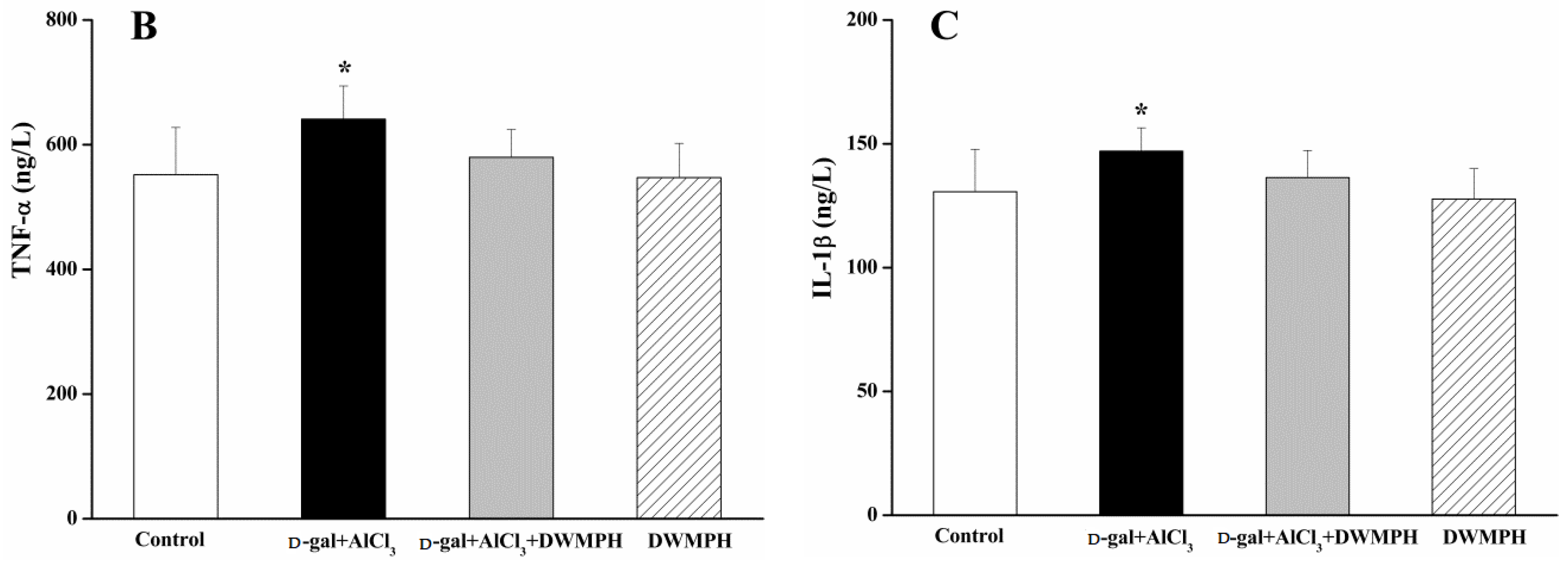
2.5. DWMPH Reduce the Expressions of Inflammatory Factors TNF-α and IL-1β
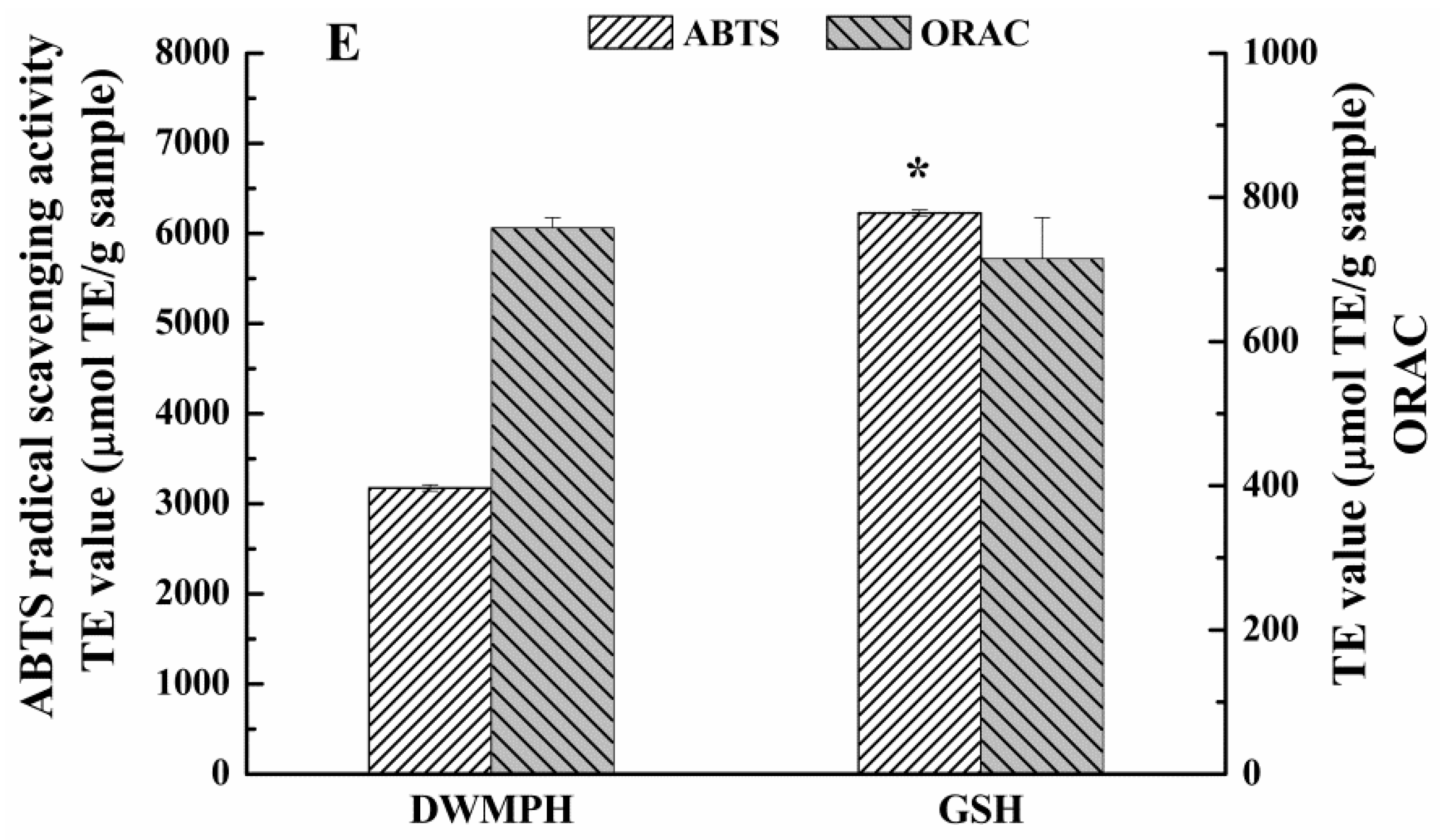
2.6. Structure and Antioxidant Activity of DWMPH
3. Materials and Methods
3.1. Materials
3.2. Preparation of Defatted Walnut Meal Protein Hydrolysates (DWMPH)
3.3. Animals and Treatments
3.4. Y-Maze Task
3.5. Morris Water Maze Test
3.6. Measurement of Cytokine, Level of Ach, and MDA, and Activities of GSH-Px, SOD, AChE, and ChAT
3.7. Hematoxylin-Eosin and Immunohistochemical Staining
3.8. Amino Acid Compositions, Secondary Structures And Molecular Weight Distribution of DWMPH
3.9. Assays of Antioxidant Activity
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- SIES, H. 1-Oxidative stress: Introductory remarks. In Oxidative Stress, 1st ed.; Sies, H., Ed.; Academic Press: London, UK, 1985; pp. 1–8. [Google Scholar]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-β1–42-induced rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res. Int. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; McGeer, E.; McGeer, P.L. Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: Implications for Alzheimer’s disease pathogenesis. Neurobiol. Aging 2015, 36, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Li, G.; Yu, J.; Zhang, L.; Wang, Y.; Wang, C.; Chen, Q. Onjisaponin B prevents cognitive impairment in a rat model of d-galactose-induced aging. Biomed. Pharmacother. 2018, 99, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, R.R.; Correa, M.C.; Gris, L.R.; Da, R.C.; Bohrer, D.; Morsch, V.M.; Schetinger, M.R. Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem. Res. 2008, 33, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Justin, T.A.; Dhivyabharathi, M.; Manivasagam, T.; Essa, M.M. Tannoid principles of Emblica officinalis attenuated aluminum chloride induced apoptosis by suppressing oxidative stress and tau pathology via Akt/GSK-3beta signaling pathway. J. Ethnopharmacol. 2016, 194, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.; Nofal, S.; Badary, O.A. Protective effects of thymoquinone on d-galactose and aluminum chloride induced neurotoxicity in rats: Biochemical, histological and behavioral changes. Neurol. Res. 2018, 40, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyah, B.; Essa, M.M.; Lee, M.; Chauhan, V.; Kaur, K.; Chauhan, A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Shukitt-Hale, B.; Cheng, V.; Joseph, J.A. Dose-dependent effects of walnuts on motor and cognitive function in aged rats. Br. J. Nutr. 2009, 101, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Effect and mechanism of oyster hydrolytic peptides on spatial learning and memory in mice. RSC Adv. 2018, 8, 6125–6135. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ren, D.; Li, J.; Fang, L.; Wang, J.; Liu, J.; Min, W. Cytoprotective effect and purification of novel antioxidant peptides from hazelnut (C. heterophylla Fisch) protein hydrolysates. J. Funct. Foods 2018, 42, 203–215. [Google Scholar] [CrossRef]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, W.; He, R.; Xiong, F.; Ma, H. Protein breakdown and release of antioxidant peptides during simulated gastrointestinal digestion and the absorption by everted intestinal sac of rapeseed proteins. LWT-Food Sci. Technol. 2017, 86, 424–429. [Google Scholar] [CrossRef]
- Orsini Delgado, M.C.; Nardo, A.; Pavlovic, M.; Rogniaux, H.; Anon, M.C.; Tironi, V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem. 2016, 197, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, T.; Zhang, J.; Xu, J.; Sun-Waterhouse, D.; Zhao, M.; Su, G. Effect of walnut protein hydrolysate on scopolamine-induced learning and memory deficits in mice. J. Food Sci. Technol. 2017, 54, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Bennett, J.J.; Tuttle, J.B. Endogenous oxidative stress in sporadic Alzheimer’s disease neuronal cybrids reduces viability by increasing apoptosis through pro-death signaling pathways and is mimicked by oxidant exposure of control cybrids. Neurobiol. Dis. 2005, 19, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dominguez, R.; Garcia-Barrera, T.; Gomez-Ariza, J.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pi, Z.; Song, F.; Liu, Z. Ginsenosides attenuate d-galactose- and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 194, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-kappaB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox. Res. 2013, 24, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Tata, A.M.; Velluto, L.; D’Angelo, C.; Reale, M. Cholinergic system dysfunction and neurodegenerative diseases: Cause or effect? CNS Neurol. Disord. Drug Targets 2014, 13, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 2015, 38, 448–458. [Google Scholar] [CrossRef] [PubMed]
- McAfoose, J.; Baune, B.T. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009, 33, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wu, D.; Zheng, Y.; Hu, B.; Zhang, Z.; Ye, Q.; Liu, C.; Shan, Q.; Wang, Y. Ursolic Acid Attenuates d-Galactose-Induced Inflammatory Response in Mouse Prefrontal Cortex through Inhibiting AGEs/RAGE/NF-kappa B Pathway Activation. Cereb. Cortex 2010, 20, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Terrando, N.; Monaco, C.; Ma, D.; Foxwell, B.M.; Feldmann, M.; Maze, M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. USA 2010, 107, 20518–20522. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, H.; Zhao, M.; Wang, X.; Yang, B.; Ren, J.; Su, G. Identification of antioxidant peptides released from defatted walnut (Juglans Sigillata Dode) meal proteins with pancreatin. LWT-Food Sci. Technol. 2015, 60, 213–220. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, J.; Zhao, H.; Jiang, W.; Guo, X.; Zhao, M.; Sun-Waterhouse, D.; Zhao, Q.; Su, G. Antioxidant and anti-acetylcholinesterase activities of anchovy (Coilia mystus) protein hydrolysates and their memory-improving effects on scopolamine-induced amnesia mice. Int. J. Food Sci. Technol. 2017, 52, 504–510. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, H.; Wang, J.; Wang, Q.; Yu, L.L.; Sun, B. Influence of extraction and solubilizing treatments on the molecular structure and functional properties of peanut protein. LWT-Food Sci. Technol. 2017, 79, 197–204. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, S.; Zhang, M.; Lin, S. Analysis of alpha-helix unfolding in the pine nut peptide Lys-Cys-His-Lys-Pro induced by pulsed electric field. J. Sci. Food Agric. 2017, 97, 4058–4065. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, H.; Li, J.; Li, Z.; Jiang, Y. Amino acid composition, molecular weight distribution and antioxidant activity of protein hydrolysates of soy sauce lees. Food Chem. 2011, 124, 551–555. [Google Scholar] [CrossRef]
- Zhao, R.J.; Huo, C.Y.; Qian, Y.; Ren, D.F.; Lu, J. Ultra-high-pressure processing improves proteolysis and release of bioactive peptides with activation activities on alcohol metabolic enzymes in vitro from mushroom foot protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Jin, D.X.; Liu, X.L.; Zheng, X.Q.; Wang, X.J.; He, J.F. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Wang, B.; Hu, F.; Wang, Y.; Zhang, B.; Deng, S.; Wu, C. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Feng, L.; Peng, F.; Wang, X.; Li, M.; Lei, H.; Xu, H. Identification and characterization of antioxidative peptides derived from simulated in vitro gastrointestinal digestion of walnut meal proteins. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Su, G.; Ren, J.; Yang, B.; Cui, C.; Zhao, M. Comparison of hydrolysis characteristics on defatted peanut meal proteins between a protease extract from Aspergillus oryzae and commercial proteases. Food Chem. 2011, 126, 1306–1311. [Google Scholar] [CrossRef]
- Duan, X.; Li, M.; Shao, J.; Chen, H.; Xu, X.; Jin, Z.; Liu, X. Effect of oxidative modification on structural and foaming properties of egg white protein. Food Hydrocoll. 2018, 75, 223–228. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Group | Initial Body Weight (g) | Final Body Weight (g) | Food Intake (g/day) |
|---|---|---|---|
| Control | 32.40 ± 1.88 | 43.10 ± 3.29 | 7.10 ± 1.24 |
| d-gal+AlCl3 | 30.84 ± 2.36 | 41.93 ± 3.91 | 7.12 ± 0.41 |
| d-gal+AlCl3+DWMPH | 31.01 ± 2.74 | 43.70 ± 4.19 | 6.50 ± 0.43 |
| DWMPH | 32.65 ± 1.97 | 41.72 ± 3.97 | 6.23 ± 0.40 |
| Amino Acid | Content (g/100 g) | Amino Acid | Content (g/100 g) | ||
|---|---|---|---|---|---|
| DWMP | DWMPH | DWMP | DWMPH | ||
| Glu | 24.239 ± 0.242a | 23.495 ± 0.616a | Lys | 2.911 ± 0.139a | 3.536 ± 0.218a |
| Arg | 15.048 ± 0.293a | 13.874 ± 0.320b | Tyr | 2.688 ± 0.203a | 2.456 ± 0.252a |
| Asp | 9.283 ± 0.569a | 11.002 ± 0.430a | Thr | 2.658 ± 0.123a | 2.956 ± 0.209a |
| Leu | 6.763 ± 0.166a | 6.624 ± 0.144a | His | 2.440 ± 0.200a | 2.278 ± 0.114a |
| Val | 5.050 ± 0.227a | 4.869 ± 0.311a | Met | 1.618 ± 0.196a | 1.270 ± 0.163a |
| Gly | 4.830 ± 0.223b | 5.650 ± 0.134a | Trp | 0.848 ± 0.064a | 1.029 ± 0.130a |
| Phe | 4.507 ± 0.178a | 4.300 ± 0.248a | Cys | 0.582 ± 0.074a | 0.473 ± 0.066a |
| Ile | 4.392 ± 0.352a | 4.430 ± 0.235a | Met + Cys | 2.199 ± 0.270a | 1.742 ± 0.229a |
| Ala | 4.152 ± 0.137a | 4.316 ± 0.165a | Phe + Tyr | 7.195 ± 0.025a | 6.753 ± 0.004b |
| Ser | 4.014 ± 0.173a | 4.592 ± 0.163a | |||
| Pro | 3.980 ± 0.243a | 2.857 ± 0.153b | EAA/TAA (%) | 28.745 ± 0.267a | 29.009 ± 0.423a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Wang, X.; Peng, F.; Liao, J.; Nai, Y.; Lei, H.; Li, M.; Xu, H. Walnut Protein Hydrolysates Play a Protective Role on Neurotoxicity Induced by d-Galactose and Aluminum Chloride in Mice. Molecules 2018, 23, 2308. https://doi.org/10.3390/molecules23092308
Feng L, Wang X, Peng F, Liao J, Nai Y, Lei H, Li M, Xu H. Walnut Protein Hydrolysates Play a Protective Role on Neurotoxicity Induced by d-Galactose and Aluminum Chloride in Mice. Molecules. 2018; 23(9):2308. https://doi.org/10.3390/molecules23092308
Chicago/Turabian StyleFeng, Li, Xiaojing Wang, Fei Peng, Jianqiao Liao, Yifan Nai, Hongjie Lei, Mei Li, and Huaide Xu. 2018. "Walnut Protein Hydrolysates Play a Protective Role on Neurotoxicity Induced by d-Galactose and Aluminum Chloride in Mice" Molecules 23, no. 9: 2308. https://doi.org/10.3390/molecules23092308