Herb–Drug Interaction Potential of Anti-Borreliae Effective Extracts from Uncaria tomentosa (Samento) and Otoba parvifolia (Banderol) Assessed In Vitro
Abstract
:1. Introduction
2. Results and Discussion
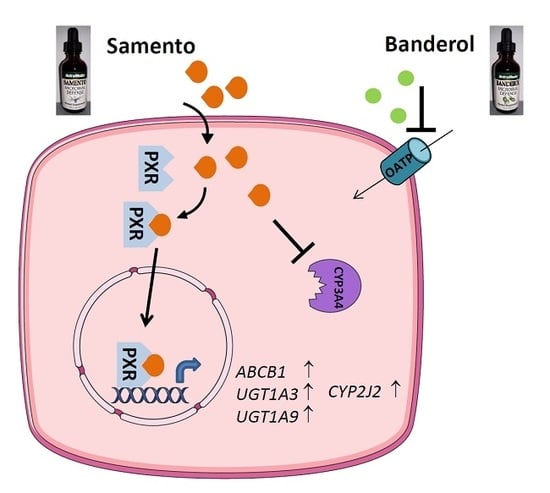
2.1. Transporter Inhibition by Samento and Banderol
2.2. Inhibition of CYP1A2, CYP2B6, CYP2C19, and CYP3A4 by Samento and Banderol
2.3. Induction of Drug Transporters and Drug Metabolizing Enzymes by Samento and Banderol
2.4. Activation of PXR and AhR
2.5. Influence of Samento on the Protein Expression of P-gp and CYP2J2
2.6. Influence of Samento on CYP2J2 mRNA Decay
2.7. Discussion
3. Materials and Methods
3.1. Materials
3.2. Stock Solutions and Rationale for the Used Dilutions
3.3. Cytotoxicity Assays
3.4. P-gp Inhibition Assay
3.5. BCRP Inhibition Assay
3.6. OATP Inhibition Assay
3.7. Inhibition of CYP1A2, CYP2B6, CYP2C19, and CYP3A4
3.8. Growth Inhibition Assay
3.9. Induction Assay
3.10. Quantification of mRNA Expression by Real-Time RT-PCR
3.11. Western Blot Analysis of P-gp and CYP2J2 Expression
3.12. PXR Reporter Gene Assay
3.13. AhR Reporter Gene Assay
3.14. Evaluation of the Influence of Samento on CYP2J2 mRNA Decay
3.15. Statistical Analysis
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Honório, I.C.G.; Bertoni, B.W.; Telles, M.P.C.; Braga, R.D.S.; França, S.C.; Coppede, J.D.S.; Correa, V.S.C.; Diniz Filho, J.A.F.; Pereira, A.M.S. Genetic and chemical diversity of Uncaria tomentosa (Willd. ex. Schult.) DC. in the Brazilian Amazon. PLoS ONE 2017, 12, e0177103. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.V.; Monagas, M.; Bartolomé, B. Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants (Basel) 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, J.J.; Xu, J.; Feng, F.; Qu, W. Medicinal uses, phytochemistry and pharmaology of the genus Uncaria. J. Ethnopharmacol. 2015, 173, 48–80. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, K.-H. Uncaria tomentosa (Willd.) D.C.: Cat‘s Claw, Uña de Gato, or Savéntaro. J. Altern. Complement. Med. 1999, 5, 143–151. [Google Scholar] [CrossRef]
- Schultes, R.E.; Raffauf, R. The Healing Forest: Medicinal and Toxic Plants of Northwest Amazonia; Dioscorides Press: Portland, OR, USA, 1990. [Google Scholar]
- Weniger, B.; Robledo, S.; Arango, G.J.; Deharo, E.; Aragón, R.; Muñoz, V.; Callapa, J.; Lobstein, A.; Anton, R. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 2001, 79, 103–200. [Google Scholar] [CrossRef]
- Datar, A.; Kaur, N.; Patel, S.; Luecke, D.; Sapi, E. In vitro effectiveness of samento and banderol herbal extracts on the different morphological forms of Borrelia burgdorferi. Townsend Lett. 2010, 7, 1–4. [Google Scholar]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2011, 379, 461–473. [Google Scholar] [CrossRef]
- Sapi, E.; Kaur, N.; Anyanwu, S.; Luecke, D.F.; Datar, A.; Patel, S.; Rossi, M.; Stricker, R.B. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect. Drug Resist. 2011, 4, 97–113. [Google Scholar]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.X.; Yi, X.L.; Si, D.Y.; Xiao, X.F.; He, X.; Li, Y.Z. Herb-drug interactions involving drug metabolizing enzymes and transporters. Curr. Drug Metab. 2011, 12, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Budzinski, J.W.; Foster, B.C.; Vandenhoek, S.; Arnason, J.T. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine 2000, 7, 273–282. [Google Scholar] [CrossRef]
- López Galera, R.M.; Ribera Pascuet, E.; Esteban Mur, J.I.; Montoro Ronsano, J.B.; Juárez Giminéz, J.C. Interaction between cat’s claw and protease inhibitors atazanavir, ritonavir and saquinavir. Eur. J. Clin. Pharmacol. 2008, 64, 1235–1236. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.D.; Roberts, E.M.; Mulford, D.J.; Yao, Y.; Ouellet, D. Oxidative metabolism of clarithromycin in the presence of human liver microsomes. Major role for the cytochrome P4503A (CYP3A) subfamily. Drug Metab. Dispos. 1997, 25, 623–630. [Google Scholar]
- Fuhr, U. Induction of drug metabolising enzymes: pharmacokinetic and toxicological consequences in humans. Clin. Pharmacokinet. 2000, 38, 493–504. [Google Scholar] [CrossRef]
- Izzo, A.A. Herb-drug interactions: An overview of the clinical evidence. Fundam. Clin. Pharmacol. 2005, 19, 1–16. [Google Scholar] [CrossRef]
- Xu, M.; Ju, W.; Hao, H.; Wang, G.; Li, P. Cytochrome P450 2J2: Distribution, function, regulation, genetic polymorphisms and clinical significance. Drug Metab. Rev. 2013, 45, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M.; Madafiglio, J.; Norris, M.D.; Haber, M. Resistance to tetracycline, a hydrophilic antibiotic, is mediated by P-glycoprotein in human multidrug-resistant cells. Biochem. Biophys. Res. Commun. 1993, 190, 79–85. [Google Scholar] [CrossRef]
- Milane, A.; Fernandez, C.; Vautier, S.; Bensimon, G.; Meininger, V.; Farinotti, R. Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood-brain barrier. J. Neurochem. 2007, 103, 164–173. [Google Scholar] [CrossRef]
- Munić, V.; Kelnerić, Z.; Mikac, L.; Eraković Haber, V. Differences in assessment of macrolide interaction with human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase activity and cellular accumulation assays. Eur. J. Pharm. Sci. 2010, 41, 86–95. [Google Scholar] [CrossRef]
- Weiss, J.; Dormann, S.M.; Martin-Facklam, M.; Kerpen, C.J.; Ketabi-Kiyanvash, N.; Haefeli, W.E. Inhibition of P-glycoprotein by newer antidepressants. J. Pharmacol. Exp. Ther. 2003, 305, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, M.; Albermann, N.; Sauer, A.; Walter-Sack, I.; Haefeli, W.E.; Weiss, J. In vitro and ex vivo evidence for modulation of P-glycoprotein activity by progestins. Biochem. Pharmacol. 2004, 68, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Rose, J.; Storch, C.H.; Ketabi-Kiyanvash, N.; Sauer, A.; Haefeli, W.E.; Efferth, T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J. Antimicrob. Chemother. 2007, 59, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Theile, D.; Spalwisz, A.; Burhenne, J.; Riedel, K.D.; Haefeli, W.E. Influence of sildenafil and tadalafil on the enzyme- and transporter-inducing effects of bosentan and ambrisentan in LS180 cells. Biochem. Pharmacol. 2013, 85, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.; Lindenmaier, H.; Haefeli, W.E.; Weiss, J. Interaction of the mitotic kinesin Eg5 inhibitor monastrol with P-glycoprotein. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 372, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Kocher, J.; Mueller, C.; Rosenzweig, S.; Theile, D. Impact of enzalutamide and its main metabolite N-desmethyl enzalutamide on pharmacokinetically important drug metabolizing enzymes and drug transporters. Biopharm. Drug Dispos. 2017, 38, 517–525. [Google Scholar] [CrossRef]
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar] [CrossRef]
- Gaedigk, A.; Baker, D.W.; Totah, R.A.; Gaedigk, R.; Pearce, R.E.; Vyhlidal, C.A.; Zeldin, D.C.; Leeder, J.S. Variability of CYP2J2 expression in human fetal tissues. J. Pharmacol. Exp. Ther. 2006, 319, 523–532. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2003, 3, RESEARCH0034. [Google Scholar]
- Zisowsky, J.; Koegel, S.; Leyers, S.; Devarakonda, K.; Kassack, M.U.; Osmak, M.; Jaehde, U. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem. Pharmacol. 2007, 73, 298–307. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Samento and Banderol are available from the authors. |






© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, J. Herb–Drug Interaction Potential of Anti-Borreliae Effective Extracts from Uncaria tomentosa (Samento) and Otoba parvifolia (Banderol) Assessed In Vitro. Molecules 2019, 24, 137. https://doi.org/10.3390/molecules24010137
Weiss J. Herb–Drug Interaction Potential of Anti-Borreliae Effective Extracts from Uncaria tomentosa (Samento) and Otoba parvifolia (Banderol) Assessed In Vitro. Molecules. 2019; 24(1):137. https://doi.org/10.3390/molecules24010137
Chicago/Turabian StyleWeiss, Johanna. 2019. "Herb–Drug Interaction Potential of Anti-Borreliae Effective Extracts from Uncaria tomentosa (Samento) and Otoba parvifolia (Banderol) Assessed In Vitro" Molecules 24, no. 1: 137. https://doi.org/10.3390/molecules24010137
APA StyleWeiss, J. (2019). Herb–Drug Interaction Potential of Anti-Borreliae Effective Extracts from Uncaria tomentosa (Samento) and Otoba parvifolia (Banderol) Assessed In Vitro. Molecules, 24(1), 137. https://doi.org/10.3390/molecules24010137