The Effects of Environmental Factors on Ginsenoside Biosynthetic Enzyme Gene Expression and Saponin Abundance
Abstract
:1. Introduction
2. Results
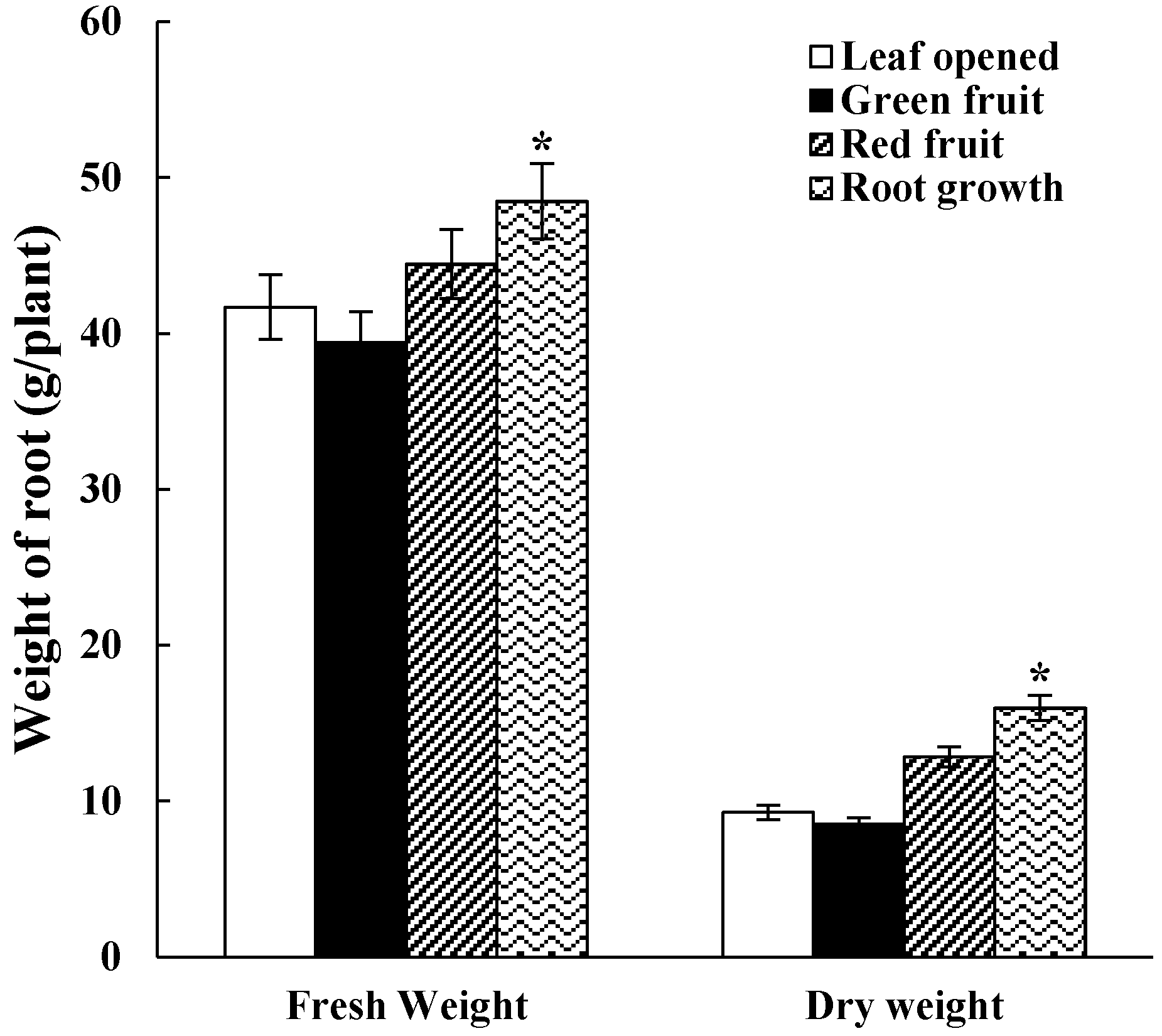
2.1. Fresh Weight and Dry Weight of Roots
2.2. Ginsenoside Content at Different Growth Times
2.3. Biosynthesis of Ginsenoside-Related Genes at Different Growth Stages
2.4. Environmental Factors
2.5. The Correlation between Individual Ginsenosides and Total Saponins
2.6. Correlation Analysis of Key Enzyme Gene Expression in the Ginsenoside Synthesis Pathway
2.7. The Correlation between Environmental Factors and Ginsenosides and Gene Expression
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Fresh and Dry Weight of Panax Ginseng
4.3. Analysis of Ginsenosides by HPLC
4.4. Extraction of RNA and Quantification of Transcript Levels
4.5. Environmental Factor Data Collection
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; The Medicine Science and Technology Press of China: Beijing, China, 2015. [Google Scholar]
- Myeong, H.J.; Tae, R.K.; Ju, O.N.; Seul, G.L. Mass production method of Korea ginseng (Panax ginseng C.A. Meyer) leaf and inhibitory effect of extracts on fat accumulation. Planta Med. Inter. Open 2017, 4, S1–S202. [Google Scholar]
- Liu, W.; Wang, Z.; Hou, J.G.; Zhou, Y.D.; He, Y.F.; Jiang, S.; Wang, Y.; Ren, S.; Li, W. The Liver Protection Effects of Maltol, a Flavoring Agent, on Carbon Tetrachloride-Induced Acute Liver Injury in Mice via Inhibiting Apoptosis and Inflammatory Response. Molecules 2018, 23, 2120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Li, G.; Jiang, C.; Yang, B.; Yang, H.J.; Xu, H.Y.; Huang, L.Q. Tissue-specific distribution of ginsenosides in different aged ginseng and antioxidant activity of ginseng leaf. Molecules 2014, 19, 17381–17399. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Rao, Z.; Cui, J.; Bao, H.; Chen, C.; Shu, C.; Gong, J.R. Ginsenosides Extracted from Nanoscale Chinese White Ginseng Enhances Anticancer Effect. J. Nanosci. Nanotechnol. 2012, 12, 1–5. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, Y.J.; Huh, J.S.; Kim, S.W.; Sohn, D.W. Improvement of erectile function by Korean red ginseng (Panax ginseng) in a male rat model of metabolic syndrome. Asian J. Androl. 2013, 15, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Kim, S.W.; Sung, J.H.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp. Gerontol. 2012, 47, 77–84. [Google Scholar] [CrossRef]
- Xie, J.T.; Mehendale, S.R.; Li, X.; Quigg, R.; Wang, X.; Wang, C.Z.; Wu, A.J.; Aung, H.H.; Rue, P.A.; Bell, G.I. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim. Biophys. Acta 2005, 1740, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Shang, W.; Yang, Y.; Zhou, L.; Jiang, B.; Jin, H.; Chen, M. Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J. Endocrinol. 2008, 198, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.K.; Johnke, R.M.; Allison, R.R.; O’brien, K.F.; Dobbs, L.J., Jr. Radioprotective potential of ginseng. Mutagenesis 2005, 20, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Tritsch, D.; Hemmerlin, A.; Bach, T.J.; Rohmer, M. Plant isoprenoid biosynthesis via the MEP pathway: In Vivo IPP/DMAPP ratio produced by (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase in tobacco BY-2 cell cultures. FEBS Lett. 2010, 584, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, M.; Rana, G.S.; Han, J. Seasonal Variation and Possible Biosynthetic Pathway of Ginsenosides in Korean Ginseng Panax ginseng Meyer. Molecules 2018, 23, 1824. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yoo, D.S.; Xu, H.; Park, N.I.; Kim, H.H.; Choi, J.E.; Park, S.U. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat. Prod. Commun. 2009, 4, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, K.; Ren, Y. Effect of light intensity on Content of Soluble Sugar, Starch and Ginseng Saponin in Ginseng Plant. J. Jilin Agric. Univ. 1994, 16, 15–17. [Google Scholar]
- Nam, M.H.; Heo, E.J.; Kim, J.Y.; Kim, S.I.; Kwon, K.H.; Seo, J.B.; Kwon, O.; Yoo, J.S.; Park, Y.M. Proteome analysis of the responses of Panax ginseng C. A. Meyer leaves to high light use of electrospray ionization quadrupole-time of flight mass spectrometry and expressed sequence tag data. Proteomics 2003, 3, 2351–2367. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.S.; Wang, X.Q.; Jing, J.R.; Ma, Q.L.; Zhang, C.C. Effect of different soil and different soil water content on photosynthetic characterristics and growth of Panax ginseng. J. Jilin Agric. Univ. 2009, 6, 725–728. [Google Scholar]
- Wang, X. Studies on Yield and Quality of Ginseng from Different Origin in Jilin Province. Master’s Thesis, Jilin Agricultural University, Jilin, China, June 2013. [Google Scholar]
- Huo, Y.; Chen, W.; Guo, X. Effect of Different Altitude on the Content of Protein in Panax Ginseng. Mod. Chin. Med. 2011, 13, 16–17. [Google Scholar]
- Yu-Jin Kim, D.Z.D.Y. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar]
- Kim, Y.J.; Lee, O.R.; Oh, J.Y.; Jang, M.G.; Yang, D.C. Functional Analysis of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Encoding Genes in Triterpene Saponin-Producing Ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.T.; Kim, S.H.; Ohyama, K.; Muranaka, T.; Choi, Y.E.; Lee, H.Y.; Kim, M.Y.; Hwang, B. Upregulation of phytosterol and triterpene biosynthesis in Centella asiatica hairy roots overexpressed ginseng farnesyl diphosphate synthase. Plant Cell Rep. 2010, 29, 403–411. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeong, J.H.; Seo, J.W.; Shin, C.G.; Kim, Y.S.; In, J.G.; Yang, D.C.; Yi, J.S.; Choi, Y.E. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; In, J.; Kwon, Y.; Choi, Y.E. Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry 2010, 71, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kwon, Y.S.; Yang, D.C.; Jung, Y.R.; Choi, Y.E. Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol. 2006, 47, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. β-amyrin synthase--cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur. J. Biochem. 1998, 256, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kim, H.J.; Kwon, Y.S.; Choi, Y.E. The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2011, 52, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, M.; Ban, Y.; Hwang, H.S.; Choi, Y.E. The involvement of β-amyrin 28-oxidase (CYP716A52v2) in oleanane-type ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2013, 54, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Hwang, H.S.; Choi, S.W.; Kim, H.J.; Choi, Y.E. Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2012, 53, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Gao, W. Advances in studies on influence of environmental factors on triterpenoid saponin synthesis in medicinal plant. Chin. Traditional Herbal Drugs 2011, 42, 398–402. [Google Scholar]
- Bian, M.; Du, X.; Wang, P.; Cui, J.; Xu, J.; Gu, J.; Zhang, T.; Chen, Y. Combination of ginsenoside Rb1 and Rd protects the retina against bright light-induced degeneration. Sci. Rep. 2017, 7, 6015. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Chi, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Irfan, M.; Kim, S.D.; Kim, S.; Oh, J.H.; Park, C.K.; Kim, H.K.; Rhee, M.H. Ginsenoside Rg3-enriched red ginseng extract inhibits platelet activation and in vivo thrombus formation. J. Ginseng Res. 2017, 41, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Shishtar, E.; Sievenpiper, J.L.; Djedovic, V.; Cozma, A.I.; Ha, V.; Jayalath, V.H.; Jenkins, D.J.; Meija, S.B.; de Souza, R.J.; Jovanovski, E. The effect of ginseng (the genus panax) on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. PLoS ONE 2014, 9, e107391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; van Hasselt, C.A.; Woo, J.K.; Chen, G.G.; Wong, Y.O.; Wang, L.H.; Leung, P.C. Effect of a Chinese herbal formula, Shi-Bi-Lin, on an experimental model of allergic rhinitis. Ann. Allergy Asthma Immunol. 2006, 96, 844–850. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.; Noh, H.; Kwon, H.; Lee, S.W.; Kim, S.Y.; Kim, Y.B. Effects of Natural Bioactive Products on the Growth and Ginsenoside Contents of Panax ginseng Cultured in an Aeroponic System. J. Ginseng Res. 2012, 36, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeon, J.; Jang, M.; Oh, J.Y.; Kwon, W.S.; Jung, S.K.; Yang, D.C. Ginsenoside profiles and related gene expression during foliation in Panax. J. Ginseng Res. 2014, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Lee, D.Y.; Yu, J.; Park, H.W.; Mo, H.S.; Park, K.C.; Hyun, D.Y.; Lee, E.H.; Kim, K.H.; Oh, C.S. Photosynthesis rates, growth, and ginsenoside contents of 2-year-old Panax ginseng grown at different light transmission rates in a greenhouse. J. Ginseng Res. 2015, 39, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Fan, Y.; Chen, Y.; Liu, D.; Cheng, H.; Gao, X.; Zhou, Y. Physiological and Biochemical Response of Panax ginseng C.A. Meyer to Drought Stress. J. Northeast Agric. Sci. 2016, 5, 37–41. [Google Scholar]
- Yang, J.; Hu, Z.; Zhang, T.; Gu, A.D.; Gong, T.; Zhu, P. Progress on the Studies of the Key Enzymes of Ginsenoside Biosynthesis. Molecules 2018, 23, 589. [Google Scholar] [CrossRef]
- Qiong, W.; Chao, S.; ShiLin, C. Identification and expression analysis of a 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from American ginseng. Plant Omics. 2012, 4, 414–420. [Google Scholar]
- Kim, Y.K.; Kim, Y.B.; Uddin, M.R.; Lee, S.; Kim, S.U.; Park, S.U. Enhanced triterpene accumulation in Panax ginseng hairy roots overexpressing mevalonate-5-pyrophosphate decarboxylase and farnesyl pyrophosphate synthase. ACS Synth. Biol. 2014, 3, 773–779. [Google Scholar] [CrossRef]
- Seo, J.W.; Jeong, J.H.; Shin, C.G.; Lo, S.C.; Han, S.S.; Yu, K.W.; Harada, E.; Han, J.-Y.; Choi, Y.-E. Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation. Phytochemistry 2005, 66, 869–877. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhu, Y.; He, M.; Zhang, Y. Molecular cloning and characterization of the gene encoding squalene epoxidase in Panax notoginseng. DNA Sequence 2008, 19, 270–273. [Google Scholar] [CrossRef]
- Chen, S.; Luo, H.; Li, Y.; Sun, Y.; Wu, Q.; Niu, Y.; Song, J.; Lv, A.; Zhu, Y.; Sun, C.; et al. 454 EST analysis detects genes putatively involved in ginsenoside biosynthesis in Panax ginseng. Plant Cell Rep. 2011, 30, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kang, S.W.; Seong, N.S.; Hyun, G.S.; Hyun, D.Y.; Kim, Y.C.; Cha, S.W. Seasonal Changes of Growth and Extract Content of Roots in Panax Ginseng C.A. Meyer. Korean J. Med. Sci. 2004, 6, 483–489. [Google Scholar]
- Liu, J.; Wang, Q.; Sun, M.; Zhu, L.; Yang, M.; Zhao, Y. Selection of reference genes for quantitative real-time PCR normalization in Panax ginseng at different stages of growth and in different organs. PLoS ONE 2014, 9, e112177. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |








| Ginsenosides | Root | Stem | Leaf | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LO | GF | RF | RG | LO | GF | RF | RG | LO | GF | RF | RG | |
| PPD/PPT | 0.95 | 1.05 | 1.52 | 1.39 | 0.23 | 0.33 | 0.27 | 0.20 | 1.22 | 1.12 | 1.03 | 1.01 |
| Rg1/total ginsenosides | 0.25 | 0.25 | 0.19 | 0.16 | 0.34 | 0.37 | 0.35 | 0.36 | 0.09 | 0.11 | 0.14 | 0.16 |
| Re/total ginsenosides | 0.19 | 0.17 | 0.15 | 0.20 | 0.43 | 0.33 | 0.41 | 0.43 | 0.25 | 0.26 | 0.27 | 0.26 |
| Rb1/total ginsenosides | 0.22 | 0.24 | 0.28 | 0.24 | 0.08 | 0.06 | 0.07 | 0.05 | 0.14 | 0.12 | 0.12 | 0.12 |
| Temperature (°C) | PAR (μE) | Relative Humidity (%) | Rain (mm) | Soil Water Potential (J/g) | |
|---|---|---|---|---|---|
| Leaf opened | 14.961c | 531.67a | 65.289c | 19.333d | −0.178b |
| Green fruit | 16.845b | 365.89c | 81.067b | 44.519b | −0.108c |
| Red fruit | 23.174a | 499.28b | 85.134a | 51.267a | −0.223a |
| Root growth | 15.314c | 343.17d | 86.609a | 24.516c | −0.289a |
| PPT | PPD | Ro | Total Saponins | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rb3 | Rd | |||
| Rg1 | 1 | 0.43 | 0.73 * | 0.31 | −0.10 | 0.27 | −0.36 | 0.29 | −0.75 * | 0.58 |
| Re | 1 | 0.91 * | 0.44 | 0.42 | 0.61 | 0.69 * | 0.36 | 0.27 | 0.72 * | |
| Rf | 1 | 0.61 | 0.46 | 0.69 * | 0.35 | 0.54 | −0.09 | 0.88 ** | ||
| Rb1 | 1 | 0.95 ** | 0.97 ** | 0.22 | 0.99 ** | 0.13 | 0.91 ** | |||
| Rc | 1 | 0.96 ** | 0.45 | 0.94 ** | 0.43 | 0.80 * | ||||
| Rb2 | 1 | 0.43 | 0.95 ** | 0.27 | 0.94 ** | |||||
| Rb3 | 1 | 0.15 | 0.88 * | 0.28 | ||||||
| Rd | 1 | 0.09 | 0.88 * | |||||||
| Ro | 1 | −0.01 | ||||||||
| Total saponins | 1 | |||||||||
| PPT | PPD | Ro | Total Saponins | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rb3 | Rd | |||
| Rg1 | 1 | 0.98 ** | 0.95 ** | 0.98 ** | 0.89 ** | 0.73 * | 0.99 ** | 1.00 ** | −0.95 ** | 0.99 ** |
| Re | 1 | 0.98 ** | 0.98 ** | 0.94 ** | 0.84 * | 0.97 ** | 0.99 ** | −0.89 ** | 1.00 ** | |
| Rf | 1 | 0.99 ** | 0.89 ** | 0.85 ** | 0.94 ** | 0.97 ** | −0.81 * | 0.99 ** | ||
| Rb1 | 1 | 0.86 ** | 0.76 * | 0.99 ** | 0.99 ** | −0.87 ** | 0.99 ** | |||
| Rc | 1 | 0.91 ** | 0.84 * | 0.90 ** | −0.83 * | 0.92 ** | ||||
| Rb2 | 1 | 0.67 | 0.76 * | −0.56 | 0.81 * | |||||
| Rb3 | 1 | 0.99 ** | −0.94 ** | 0.98 ** | ||||||
| Rd | 1 | −0.93 ** | 0.99 ** | |||||||
| Ro | 1 | −0.89 ** | ||||||||
| Total saponins | 1 | |||||||||
| HMGR | SS | FPS | SE | DS | PPDS | PPTS | β-AS | OAS | |
|---|---|---|---|---|---|---|---|---|---|
| HMGR | 1 | 0.59 | 0.89 ** | 0.31 | −0.87 ** | −0.06 | −0.99 ** | 0.74 * | −0.67 * |
| SS | 1 | 0.21 | 0.85 ** | −0.78 * | −0.02 | −0.58 | 0.27 | −0.08 | |
| FPS | 1 | −0.16 | −0.55 | −0.29 | −0.92 ** | 0.59 | −0.59 | ||
| SE | 1 | −0.72 * | 0.46 | −0.23 | 0.36 | −0.19 | |||
| DS | 1 | −0.32 | 0.79 * | −0.81 ** | 0.68 * | ||||
| PPDS | 1 | 0.22 | 0.59 | −0.60 | |||||
| PPTS | 1 | −0.63 | 0.55 | ||||||
| β-AS | 1 | −0.98 ** | |||||||
| OAS | 1 |
| HMGR | SS | FPS | SE | DS | PPDS | PPTS | β-AS | OAS | |
|---|---|---|---|---|---|---|---|---|---|
| HMGR | 1 | 0.32 | 0.91 ** | 0.61 | 0.77 * | 0.33 | −0.77 * | 0.27 | −0.06 |
| SS | 1 | 0.54 | 0.93 ** | 0.85 * | 0.96 ** | −0.28 | 0.99 ** | 0.90 ** | |
| FPS | 1 | 0.81 * | 0.85 * | 0.62 | −0.92 ** | 0.54 | 0.26 | ||
| SE | 1 | 0.96 ** | 0.95 ** | −0.60 | 0.93 ** | 0.75 * | |||
| DS | 1 | 0.82 * | −0.59 | 0.81 * | 0.56 | ||||
| PPDS | 1 | −0.46 | 0.99 ** | 0.92 ** | |||||
| PPTS | 1 | −0.33 | -0.12 | ||||||
| β-AS | 1 | 0.94 ** | |||||||
| OAS | 1 |
| Temperature (°C) | PAR (μE) | Relative Humidity (%) | Rain (mm) | Soil Water Potential (J/g) | |
|---|---|---|---|---|---|
| Rg1 | 0.07 | 0.91 ** | −0.85 ** | −0.13 | 0.45 |
| Re | −0.23 | 0.58 | −0.51 | −0.71 * | −0.55 |
| Rf | 0.03 | 0.87 ** | −0.65 | −0.46 | −0.26 |
| Rb1 | 0.77 * | 0.66 | 0.12 | 0.31 | −0.45 |
| Rc | 0.71 * | 0.41 | 0.36 | 0.26 | −0.69 * |
| Rb2 | 0.62 | 0.64 | 0.07 | 0.10 | −0.60 |
| Rb3 | −0.28 | −0.12 | 0.16 | −0.62 | −0.94 ** |
| Rd | 0.83 ** | 0.64 | 0.17 | 0.40 | −0.40 |
| Ro | −0.10 | −0.49 | 0.60 | −0.28 | −0.92 ** |
| PPT | −0.03 | 0.93 ** | −0.84 ** | −0.40 | 0.07 |
| PPD | 0.73 * | 0.54 | 0.23 | 0.26 | −0.59 |
| Total saponins | 0.60 | 0.76 * | −0.08 | 0.08 | −0.48 |
| Temperature (°C) | PAR (μE) | Relative Humidity (%) | Rain (mm) | Soil Water Potential (J/g) | |
|---|---|---|---|---|---|
| Rg1 | 0.40 | −0.44 | 0.89 ** | 0.26 | −0.79 * |
| Re | 0.53 | −0.43 | 0.94 ** | 0.43 | −0.67 |
| Rf | 0.66 | −0.27 | 0.89 ** | 0.51 | −0.64 |
| Rb1 | 0.56 | −0.28 | 0.86 ** | 0.36 | −0.76 * |
| Rc | 0.41 | −0.67 | 1.00 ** | 0.52 | −0.44 |
| Rb2 | 0.70 | −0.43 | 0.93 ** | 0.83 ** | −0.17 |
| Rb3 | 0.41 | −0.35 | 0.83 ** | 0.21 | −0.84 ** |
| Rd | 0.45 | −0.24 | 0.89 ** | 0.31 | −0.77 |
| Ro | −0.09 | 0.60 | −0.81 ** | 0.00 | 0.83 ** |
| PPT | −0.03 | 0.93 ** | −0.84 ** | −0.40 | 0.07 |
| PPD | 0.73 * | 0.54 | 0.23 | 0.26 | −0.59 |
| Total saponins | 0.73 * | 0.04 | 0.69 * | 0.42 | −0.72 * |
| Temperature (°C) | PAR (μE) | Relative Humidity (%) | Rain (mm) | Soil Water Potential (J/g) | |
|---|---|---|---|---|---|
| HMGR | −0.24 | 0.82 ** | −0.96 ** | −0.47 | 0.30 |
| SS | 0.62 | 0.94 ** | −0.35 | 0.21 | −0.02 |
| FPS | −0.64 | 0.50 | −0.94 ** | −0.81 ** | 0.13 |
| SE | 0.82 ** | 0.73 * | −0.12 | 0.65 | 0.33 |
| DS | −0.19 | −0.91 ** | 0.78 * | −0.03 | −0.52 |
| PPDS | 0.27 | −0.03 | −0.02 | 0.67 | 0.91 ** |
| PPTS | 0.28 | −0.81 ** | 0.93 ** | 0.57 | −0.13 |
| β-AS | −0.21 | 0.50 | −0.82 ** | −0.07 | 0.86 ** |
| OAS | 0.36 | −0.33 | 0.79 * | 0.14 | −0.88 ** |
| Temperature (°C) | PAR (μE) | Relative Humidity (%) | Rain (mm) | Soil Water Potential (J/g) | |
|---|---|---|---|---|---|
| HMGR | −0.24 | 0.44 | −0.37 | −0.72 * | −0.69 * |
| SS | −0.43 | 0.65 | −1.00 ** | −0.52 | 0.46 |
| FPS | −0.60 | 0.31 | −0.58 | −0.94 ** | −0.46 |
| SE | −0.59 | 0.57 | −0.95 ** | −0.78 * | 0.12 |
| DS | −0.38 | 0.71 * | −0.85 ** | −0.72 * | −0.07 |
| PPDS | −0.66 * | 0.43 | −0.97 ** | −0.69 * | 0.41 |
| PPTS | 0.77 * | 0.10 | 0.33 | 0.96 ** | 0.54 |
| β-AS | −0.55 | 0.53 | −0.99 ** | −0.58 | 0.49 |
| OAS | −0.54 | 0.34 | −0.89 ** | −0.38 | 0.74 * |
| HMGR | SS | FPS | SE | DS | PPDS | PPTS | β-AS | OAS | |
|---|---|---|---|---|---|---|---|---|---|
| Rg1 | 0.25 | 0.87 ** | 0.28 | 0.71 * | 0.74 * | 0.70 * | 0.10 | 0.79 * | 0.69 * |
| Re | 0.98 ** | 0.48 | 0.91 ** | 0.72 * | 0.87 ** | 0.46 | −0.71 * | 0.42 | 0.09 |
| Rf | 0.82 ** | 0.63 | 0.72 * | 0.74 * | 0.90 ** | 0.51 | −0.39 | 0.54 | 0.24 |
| Rb1 | 0.43 | −0.12 | 0.04 | −0.09 | 0.19 | −0.34 | 0.23 | −0.27 | −0.47 |
| Rc | 0.47 | −0.37 | 0.06 | −0.25 | 0.04 | −0.53 | 0.09 | −0.49 | −0.71 * |
| Rb2 | 0.61 | −0.08 | 0.25 | 0.03 | 0.32 | −0.26 | 0.00 | −0.22 | −0.48 |
| Rb3 | 0.81 ** | −0.21 | 0.71 * | 0.17 | 0.30 | −0.10 | −0.80 ** | −0.21 | −0.47 |
| Rd | 0.34 | −0.16 | −0.05 | −0.16 | 0.12 | −0.39 | 0.31 | −0.31 | −0.49 |
| Ro | 0.44 | −0.64 | 0.30 | −0.31 | −0.20 | −0.51 | −0.53 | 0.62 | 0.77 * |
| PPT | 0.62 | 0.83 ** | 0.60 | 0.83 ** | 0.92 ** | 0.69 * | −0.24 | 0.74 * | 0.52 |
| PPD | 0.48 | −0.24 | 0.08 | −0.16 | 0.14 | −0.43 | 0.13 | −0.38 | −0.60 |
| Total saponins | 0.61 | 0.07 | 0.27 | 0.14 | 0.42 | −0.13 | 0.03 | −0.07 | −0.34 |
| HMGR | SS | FPS | SE | DS | PPDS | PPTS | B-AS | OAS | |
|---|---|---|---|---|---|---|---|---|---|
| Rg1 | 0.11 | −0.90 ** | −0.18 | −0.71 * | −0.55 | −0.88 ** | −0.01 | −0.92 ** | −0.99 ** |
| Re | −0.04 | −0.95 ** | −0.34 | −0.81 ** | −0.65 | −0.95 ** | 0.17 | −0.97 ** | −0.99 ** |
| Rf | −0.03 | −0.90 ** | −0.37 | −0.80 ** | −0.60 | −0.95 ** | 0.26 | −0.95 ** | −0.99 ** |
| Rb1 | 0.12 | −0.87 ** | −0.22 | −0.71 * | −0.51 | −0.90 ** | 0.10 | −0.92 ** | −1.00 ** |
| Rc | −0.34 | −1.00 ** | −0.54 | −0.93 ** | −0.86 ** | −0.95 ** | 0.28 | −0.98 ** | −0.89 ** |
| Rb2 | −0.55 | −0.91 ** | −0.80 ** | −0.99 ** | −0.90 ** | −0.97 ** | 0.65 | −0.93 ** | −0.79 ** |
| Rb3 | 0.21 | −0.85 ** | −0.09 | −0.63 | −0.45 | −0.83 ** | −0.06 | −0.88 ** | −0.98 ** |
| Rd | 0.08 | −0.91 ** | −0.21 | −0.73 * | −0.56 | −0.90 ** | 0.04 | −0.93 ** | −0.99 ** |
| Ro | −0.19 | 0.84 ** | −0.01 | 0.58 | 0.47 | 0.73 * | 0.27 | 0.82 ** | 0.89 ** |
| PPT | 0.62 | 0.83 ** | 0.60 | 0.83 ** | 0.92 ** | 0.69 | −0.24 | 0.74 * | 0.52 |
| PPD | 0.48 | −0.24 | 0.08 | −0.16 | 0.14 | −0.43 | 0.13 | −0.38 | −0.60 |
| Total saponins | 0.22 | −0.70 * | −0.18 | −0.58 | −0.32 | −0.81 ** | 0.19 | −0.79 * | −0.92 ** |
| − | Standard Curve Equation | R2 | Linear Range (μg) |
|---|---|---|---|
| Ro | y = 2796.01284 x − 40.64132 | 0.99969 | 6.0~0.375 |
| Rg1 | y = 3131.42174 x − 34.75556 | 0.99943 | 6.0~0.375 |
| Re | y = 3097.80287 x − 35.99583 | 0.99945 | 6.0~0.375 |
| Rf | y = 3614.44564 x − 32.36528 | 0.99912 | 6.0~0.375 |
| Rb1 | y = 2246.85066 x − 17.05278 | 0.99989 | 6.0~0.375 |
| Rc | y = 2528.59020 x − 19.43056 | 0.99985 | 6.0~0.375 |
| Rb2 | y = 2668.61410 x − 36.21944 | 0.99941 | 6.0~0.375 |
| Rb3 | y = 3451.35006 x − 47.50556 | 0.99932 | 6.0~0.375 |
| Rd | y = 3009.68339 x − 30.33472 | 0.99967 | 6.0~0.375 |
| Gene Name | Accession No. | Primer (5′−3′) | Reference |
|---|---|---|---|
| GAPDH | KF699323 | F: ATGGACCATCAGCAAAGGAC R: GGTAGCACTTTCCCAACAGC | Liu J et al. [48] |
| HMGR | GQ455990 | F: TTGGATTGAAGGGCGAGGAAAG R: CAGCAACAGCAGAACCAGCAAG | Kim et al. (2014b) [21] |
| FPS | DQ087959 | F: CAAGAAGCATTTCCGACAA R: CTCTCCTACAAGGGTGGTGA | Kim et al. (2010) [22] |
| SS | AB115496 | F: GGACTTGTTGGATTAGGGTTG R: ACTGCCTTGGCTGAGTTTTC | Lee et al. (2004) [23] |
| SE | AB122078 | F: ATGCTTTGAATATGCGCCATC R: CATGGAGATCGCGTAAAGGTC | Han et al. (2010) [24] |
| DS | AB122080 | F: ACCGCCGTTGAGATTAGATG R: ATAGGGCAATGATAAGGGGAG | Han et al. (2006) [25] |
| β-AS | AB009030 | F: GCGGAAGGGAATAAGATGAC R: CTCAGCTCTCCGGACAGC | Kushiro et al. (1998a) [26] |
| CYP716A52v2(OAS) | JX036032 | F: AGGAGCAAATGGAGATAG R: AACCGTTGTAGGTGAAAT | Han et al. (2013) [28] |
| CYP716A47(PPDS) | JN604537 | F: TCACCTTCGTTCTCAACTATC R: TCTTCCTCAAATCCTCCCAAT | Han et al. (2011) [27] |
| CYP716A53v2(PPTS) | JX036031 | F: ATCGGACAACGAGGCAGCAC R: GCCAACAGGCCAACTCAA | Han et al. (2012) [29] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Han, M.; Yang, L.; Han, Z.; Cheng, L.; Sun, Z.; Yang, L. The Effects of Environmental Factors on Ginsenoside Biosynthetic Enzyme Gene Expression and Saponin Abundance. Molecules 2019, 24, 14. https://doi.org/10.3390/molecules24010014
Zhang T, Han M, Yang L, Han Z, Cheng L, Sun Z, Yang L. The Effects of Environmental Factors on Ginsenoside Biosynthetic Enzyme Gene Expression and Saponin Abundance. Molecules. 2019; 24(1):14. https://doi.org/10.3390/molecules24010014
Chicago/Turabian StyleZhang, Tao, Mei Han, Limin Yang, Zhongming Han, Lin Cheng, Zhuo Sun, and Linlin Yang. 2019. "The Effects of Environmental Factors on Ginsenoside Biosynthetic Enzyme Gene Expression and Saponin Abundance" Molecules 24, no. 1: 14. https://doi.org/10.3390/molecules24010014





