Protective Effect of Phenolic Compounds Isolated from Mugwort (Artemisia argyi) against Contrast-Induced Apoptosis in Kidney Epithelium Cell Line LLC-PK1
Abstract
:1. Introduction
2. Results
2.1. Protective Effect of Phenolic Compounds Isolated from A. argyi on Iodixanol-Induced Renal Proximal Tubular LLC-PK1 Cell Death
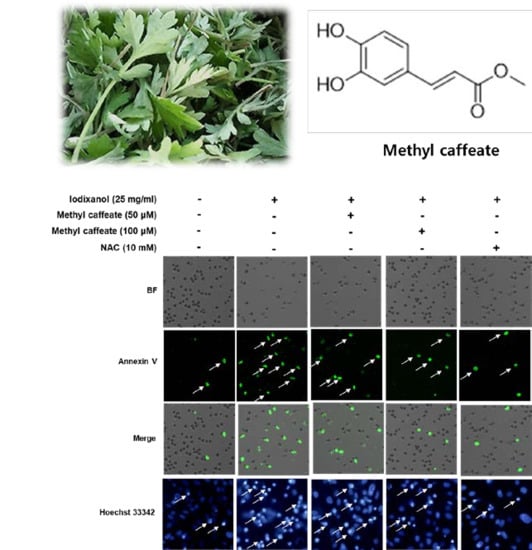
2.2. Effect of Methyl Caffeate on Iodixanol-Induced Apoptosis and ROS Generation in LLC-PK1 Cells
2.3. Effect of Methyl Caffeate on Expression Levels of MAP Kinase (JNK, ERK and P38), Kidney Injury Molecule-1 (KIM-1) and Cleaved Caspase-3 on Iodixanol-Treated LLC-PK1 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. General Analytical Procedures
4.3. Plant Material
4.4. Extraction and Isolation of Phenolic Compounds from A. argyi
4.5. Cell Culture
4.6. Quantification of Cellular Viability with Ez-Cytox Assay
4.7. Quantification of DNA Fragmentation by Hoechst 33342 Staining
4.8. Quantification of ROS Level by DCFH-DA Staining
4.9. Quantification of Apoptosis with Image-Based Cytometric Assay
4.10. Western Blotting Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. Biomed. Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Kuhlmann, M.K.; Grgic, A.; Heckmann, M.; Kramann, B.; Uder, M. Cytotoxic effects of ionic high-osmolar, nonionic monomeric, and nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology 2005, 235, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.H.; Kang, E.; Park, S.; Jo, H.A.; Lee, H.; Kim, S.M.; Lee, J.P.; Oh, K.H.; Joo, K.W.; et al. Contrast-induced nephropathy after computed tomography in stable CKD patients with proper prophylaxis: 8-year experience of outpatient prophylaxis program. Medicine 2016, 95, e3560. [Google Scholar] [CrossRef] [PubMed]
- Briguori, C.; Donnarumma, E.; Quintavalle, C.; Fiore, D.; Condorelli, G. Contrast-induced acute kidney injury: Potential new strategies. Curr. Opin. Nephrol. Hypertens 2015, 24, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, C.; Brenca, M.; De Micco, F.; Fiore, D.; Romano, S.; Romano, M.F.; Apone, F.; Bianco, A.; Zabatta, M.A.; Troncone, G.; et al. In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis. 2011, 2, e155. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Thompson, C.A. Contrast-induced acute kidney injury: The at-risk patient and protective measures. Curr. Cardiol. Rep. 2010, 12, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Chow, W.H.; Chan, T.M.; Lo, S.K.; Kwok, O.H.; Yip, A.; Fan, K.; Lee, C.H.; Lam, W.F. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: A randomized controlled trial. JAMA 2003, 289, 553–558. [Google Scholar] [CrossRef]
- Romano, G.; Briguori, C.; Quintavalle, C.; Zanca, C.; Rivera, N.V.; Colombo, A.; Condorelli, G. Contrast agents and renal cell apoptosis. Eur. Heart J. 2008, 29, 2569–2576. [Google Scholar] [CrossRef] [Green Version]
- Idee, J.M.; Bourbouze, R. Effects of iodinated X-ray contrast media on renal epithelial cells in culture. Investig. Radiol. 1995, 30, 323–324. [Google Scholar] [CrossRef]
- Lee, H.C.; Sheu, S.H.; Liu, I.H.; Lee, C.C.; Hsieh, C.C.; Yen, H.W.; Lai, W.T.; Chang, J.G. Impact of short-duration administration of N-acetylcysteine, probucol and ascorbic acid on contrast-induced cytotoxicity. J. Nephrol. 2012, 25, 56–62. [Google Scholar] [CrossRef]
- Tasanarong, A.; Piyayotai, D.; Thitiarchakul, S. Protection of radiocontrast induced nephropathy by vitamin E (alpha tocopherol): A randomized controlled pilot study. J. Med. Assoc. Thai. 2009, 92, 1273–1281. [Google Scholar] [PubMed]
- Tasanarong, A.; Vohakiat, A.; Hutayanon, P.; Piyayotai, D. New strategy of α-and γ-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrol. Dial. Transplant. 2013, 28, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.; Ming, T.W.; Aung, H.P.; Hao, S.J. Preliminary screening of Artemisia argyi for antioxidant potentials. Int. J. Pharmacog. Phytochem. Res. 2016, 8, 347–355. [Google Scholar]
- Huang, H.C.; Wang, H.F.; Yih, K.H.; Chang, L.Z.; Chang, T.M. Dual bioactivities of essential oil extracted from the leaves of Artemisia argyi as an antimelanogenic versus antioxidant agent and chemical composition analysis by GC/MS. Int. J. Mol. Sci. 2012, 13, 14679–14697. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jung, Y.; Kim, S.N. Identification of eupatilin from Artemisia argyi as a selective PPARα agonist using affinity selection ultrafiltration LC-MS. Molecules 2015, 20, 13753–13763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Lv, J.L.; Chen, H.L.; Yan, X.Q.; Duan, J.A. Chemical constituents from Artemisia argyi and their chemotaxonomic significance. Biochem. Syst. Ecol. 2013, 50, 455–458. [Google Scholar] [CrossRef]
- Park, J.M.; Han, Y.M.; Lee, J.S.; Ko, K.H.; Hong, S.P.; Kim, E.H.; Hahm, K.B. Nrf2-mediated mucoprotective and anti-inflammatory actions of Artemisia extracts led to attenuate stress related mucosal damages. J. Clin. Biochem. Nutr. 2015, 56, 132–142. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Yang, W.; Meng, D. Gastro-protective effect of edible plant Artemisia argyi in ethanol-induced rats via normalizing inflammatory responses and oxidative stress. J. Ethnopharmacol. 2018, 214, 207–217. [Google Scholar] [CrossRef]
- Ryu, B.K.; Ahn, B.O.; Oh, T.Y.; Kim, S.H.; Kim, W.B.; Lee, E.B. Studies on protective effect of DA-9601, Artemisia asiatica extract, on acetaminophen-and CCI 4-induced liver damage in rats. Arch. Pharm. Res. 1998, 21, 508–513. [Google Scholar] [CrossRef]
- Hahm, K.B.; Kim, J.H.; You, B.M.; Kim, Y.S.; Cho, S.W.; Yim, H.; Ahn, B.O.; Kim, W.B. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas 1998, 17, 153–157. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.K.; Jeon, S.B.; Son, K.H.; Kim, E.H.; Kang, S.K.; Sung, N.D.; Kwon, B.M. New sesquiterpene-monoterpene lactone, artemisolide, isolated from Artemisia argyi. Tetrahedron Lett. 2002, 43, 6205–6208. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.K.; Seo, J.M.; Kang, H.M.; Kim, J.H.; Son, K.H.; Lee, H.; Kwon, B.M.; Shin, J.; Seo, Y. Arteminolides B, C, and D, new inhibitors of farnesyl protein transferase from Artemisia a rgyi. J. Org. Chem. 2002, 67, 7670–7675. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [PubMed]
- Lao, A.; Fujimoto, Y.; Tatsuno, T. Studies on the constituents of Artemisia argyi Levl et Vant. Chem. Pharm. Bull. 1984, 32, 723–727. [Google Scholar] [CrossRef]
- Tan, R.; Jia, Z. Eudesmanolides and other constituents from Artemisia argyi. Planta Med. 1992, 58, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Lu, G.R.; Meng, D.L.; Li, N.; Li, X. Chemical constituents from the Folium Artemisiae Argyi (II). J. Shenyang Pharm. Univ. 2010, 27, 548. [Google Scholar]
- Nakasugi, T.; Nakashima, M.; Komai, K. Antimutagens in gaiyou (Artemisia argyi Levl. et Vant.). J. Agric. Food Chem. 2000, 48, 3256–3266. [Google Scholar] [CrossRef]
- Lee, D.; Kim, C.E.; Park, S.Y.; Kim, K.O.; Hiep, N.T.; Lee, D.; Jang, H.J.; Lee, J.W.; Kang, K.S. Protective effect of Artemisia argyi and its flavonoid constituents against contrast-induced cytotoxicity by iodixanol in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19, 1387. [Google Scholar] [CrossRef]
- Haller, C.; Hizoh, I. The cytotoxicity of iodinated radiocontrast agents on renal cells in vitro. Investig. Radiol. 2004, 39, 149–154. [Google Scholar] [CrossRef]
- Ueda, J.; Nygren, A.; Sjoquist, M.; Jacobsson, E.; Ulfendahl, H.R.; Araki, Y. Iodine concentrations in the rat kidney measured by X-ray microanalysis. Comparison of concentrations and viscosities in the proximal tubules and renal pelvis after intravenous injections of contrast media. Acta Radiol. 1998, 39, 90–95. [Google Scholar]
- Schmeda-Hirschmann, G.; Tapia, A.; Theoduloz, C.; Rodriguez, J.; Lopez, S.; Feresin, G.E. Free radical scavengers and antioxidants from Tagetes mendocina. Z Naturforsch. C 2004, 59, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Ding, H.Y.; Hung, W.J.; Liang, C.H. Antioxidative characteristics and inhibition of α-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Dermatol. 2010, 19, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, J.; Zhang, J.; Peng, J.; Liu, T.; Xin, Z. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015, 171, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Gao, Z.; Yu, B.; Zheng, P.; He, J.; Yu, J.; Huang, Z.; Chen, D.; Mao, X. Effects of benzoic acid (VevoVitall®) on the performance and jejunal digestive physiology in young pigs. J. Anim. Sci. Biotechnol. 2016, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, C.; Fiore, D.; De Micco, F.; Visconti, G.; Focaccio, A.; Golia, B.; Ricciardelli, B.; Donnarumma, E.; Bianco, A.; Zabatta, M.A.; et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation 2012, 126. [Google Scholar] [CrossRef]
- Akdeniz, D.; Celik, H.T.; Kazanci, F.; Yilmaz, H.; Yalcin, S.; Bilgic, M.A.; Ruzgaresen, N.; Akcay, A.; Eryonucu, B. Is kidney injury molecule 1 a valuable tool for the early diagnosis of contrast-induced nephropathy? J. Investig. Med. 2015, 63, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Vijayasimha, M.; Padma, V.V.; Mujumdar, S.K.D.; Satyanarayana, P.; Yadav, A. Kidney injury molecule-1: A urinary biomarker for contrast-induced acute kidney injury. Med. J. Dr. DY Patil Vidyapeeth. 2014, 7, 321. [Google Scholar] [CrossRef]
- Vacotto, M.; Coso, O.; de Plazas, S.F. Programmed cell death and differential JNK, p38 and ERK response in a prenatal acute hypoxic hypoxia model. Neurochem. Int. 2008, 52, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.K.; Chang, J.Y. Oxidative stress causes ERK phosphorylation and cell death in cultured retinal pigment epithelium: Prevention of cell death by AG126 and 15-deoxy-delta 12, 14-PGJ 2. BMC Ophthalmol. 2003, 3, 5. [Google Scholar] [CrossRef]
- Hongmei, Z. Extrinsic and intrinsic apoptosis signal pathway review. In Apoptosis and Medicine; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Okuyama, E.; Hasegawa, T.; Matsushita, T.; Fujimoto, H.; Ishibashi, M.; Yamazaki, M. Analgesic components of saposhnikovia root (Saposhnikovia divaricata). Chem. Pharm. Bull. 2001, 49, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Basabe, P.; de Román, M.; Marcos, I.S.; Diez, O.; Blanco, A.; Bodero, O.; Mollinedo, F.; Sierra, B.G.; Urones, J.G. Prenylflavonoids and prenyl/alkyl-phloroacetophenones: Synthesis and antitumour biological evaluation. Eur. J. Med. Chem. 2010, 45, 4258–4269. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.H.; Nagle, A.A.; Zhang, Y.; Scarmagnani, S.; Palaniappan, P.; Bradshaw, T.D.; Holmgren, A.; Westwell, A.D. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: Potential candidates for cancer therapy and chemoprevention. Free Radic. Biol. Med. 2010, 48, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M.; Scragg, J.T.; Galofre, A.M.; Gui, X.C.; Lewis, W. A concise synthesis of honokiol. Tetrahedron. 2010, 66, 8029–8035. [Google Scholar] [CrossRef]
- Silveira-Dorta, G.; Monzon, D.M.; Crisostomo, F.P.; Martin, T.; Martin, V.S.; Carrillo, R. Oxidation with air by ascorbate-driven quinone redox cycling. Chem. Commun. 2015, 51, 7027–7030. [Google Scholar] [CrossRef] [Green Version]
- Ito, C.; Furukawa, H.; Ishii, H.; Ishikawa, T.; Haginiwa, J. The chemical composition of murraya panicdata. The structure of five new coumarins and one new alkaloid and the stereochemistry of murrangatin and related coumarins. J. Chem. Soc. Perkin Trans. 1990, 2047–2055. [Google Scholar] [CrossRef]
- Hage, R.; De Boer, J.W.; Saisaha, P. Method for the Oxidation of Unsaturated Organic Compounds. WO2011GB01093, 21 July 2011. [Google Scholar]
- Sheng, Z.; Dai, H.; Pan, S.; Wang, H.; Hu, Y.; Ma, W. Isolation and characterization of an α-glucosidase inhibitor from Musa spp. (Baxijiao) flowers. Molecules 2014, 19, 10563–10573. [Google Scholar] [CrossRef]
- Kerkatou, M.; Redouane-Salah, A.; León, F.; Brouard, I.; Mosset, P.; Menad, A.; Ameddah, S.; Benayache, S.; Bermejo, J.; Benayache, F. Secondary metabolites and antioxidant activity of Limonium duriusculum (de Girard) kuntze extracts. Asian J. Chem. 2016, 28, 2695–2700. [Google Scholar] [CrossRef]
- Uwai, K.; Osanai, Y.; Imaizumi, T.; Kanno, S.; Takeshita, M.; Ishikawa, M. Inhibitory effect of the alkyl side chain of caffeic acid analogues on lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophages. Bioorg. Med. Chem. 2008, 16, 7795–7803. [Google Scholar] [CrossRef]
- Kowczyk-Sadowy, M.; Swislocka, R.; Lewandowska, H.; Piekut, J.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H- and 13C-NMR), theoretical and microbiological study of trans o-coumaric acid and alkali metal o-coumarates. Molecules 2015, 20, 3146–3169. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yu, J.Q. Pd(II)-catalyzed hydroxylation of arenes with 1 atm of O(2) or air. J. Am. Chem. Soc. 2009, 131, 14654–14655. [Google Scholar] [CrossRef]
- Nemoto, K.; Tanaka, S.; Konno, M.; Onozawa, S.; Chiba, M.; Tanaka, Y.; Sasaki, Y.; Okubo, R.; Hattori, T. Me2AlCl-mediated carboxylation, ethoxycarbonylation, and carbamoylation of indoles. Tetrahedron 2016, 72, 734–745. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.M.; Jung, K.; Chin, Y.W.; Kang, K.S. α-mangostin improves insulin secretion and protects INS-1 cells from streptozotocin-induced damage. Int. J. Mol. Sci. 2018, 19, 1484. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Hong, Y.D.; Baek, K.S.; Yoo, S.; Hong, Y.H.; Kim, J.H.; Lee, J.O.; Kim, D.; Park, J.; Cho, J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guon, T.; Chung, H.S. Induction of apoptosis with Moringa oleifera fruits in HCT116 human colon cancer cells via intrinsic pathway. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Park, J.Y.; Kang, K.S.; Park, J.H.; Hwang, G.S. Processed Panax ginseng, sun ginseng, inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans. J. Ginseng Res. 2017, 41, 257–267. [Google Scholar] [CrossRef]
- Yoon, D.H.; Han, C.; Fang, Y.; Gundeti, S.; Lee, I.-S.H.; Song, W.O.; Hwang, K.-C.; Kim, T.W.; Sung, G.-H.; Park, H. Inhibitory activity of cordyceps bassiana extract on LPS-induced inflammation in RAW 264.7 cells by suppressing NF-κB activation. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.O.; Lee, D.; Hiep, N.T.; Song, J.H.; Lee, H.-J.; Lee, D.; Kang, K.S. Protective Effect of Phenolic Compounds Isolated from Mugwort (Artemisia argyi) against Contrast-Induced Apoptosis in Kidney Epithelium Cell Line LLC-PK1. Molecules 2019, 24, 195. https://doi.org/10.3390/molecules24010195
Kim KO, Lee D, Hiep NT, Song JH, Lee H-J, Lee D, Kang KS. Protective Effect of Phenolic Compounds Isolated from Mugwort (Artemisia argyi) against Contrast-Induced Apoptosis in Kidney Epithelium Cell Line LLC-PK1. Molecules. 2019; 24(1):195. https://doi.org/10.3390/molecules24010195
Chicago/Turabian StyleKim, Kem Ok, Dahae Lee, Nguyen Tuan Hiep, Ji Hoon Song, Hae-Jeung Lee, Dongho Lee, and Ki Sung Kang. 2019. "Protective Effect of Phenolic Compounds Isolated from Mugwort (Artemisia argyi) against Contrast-Induced Apoptosis in Kidney Epithelium Cell Line LLC-PK1" Molecules 24, no. 1: 195. https://doi.org/10.3390/molecules24010195
APA StyleKim, K. O., Lee, D., Hiep, N. T., Song, J. H., Lee, H.-J., Lee, D., & Kang, K. S. (2019). Protective Effect of Phenolic Compounds Isolated from Mugwort (Artemisia argyi) against Contrast-Induced Apoptosis in Kidney Epithelium Cell Line LLC-PK1. Molecules, 24(1), 195. https://doi.org/10.3390/molecules24010195