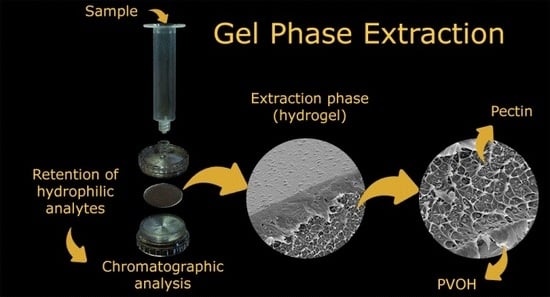
Evaluation of Polyvinyl Alcohol/Pectin-Based Hydrogel Disks as Extraction Phase for Determination of Steroidal Hormones in Aqueous Samples by GC-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
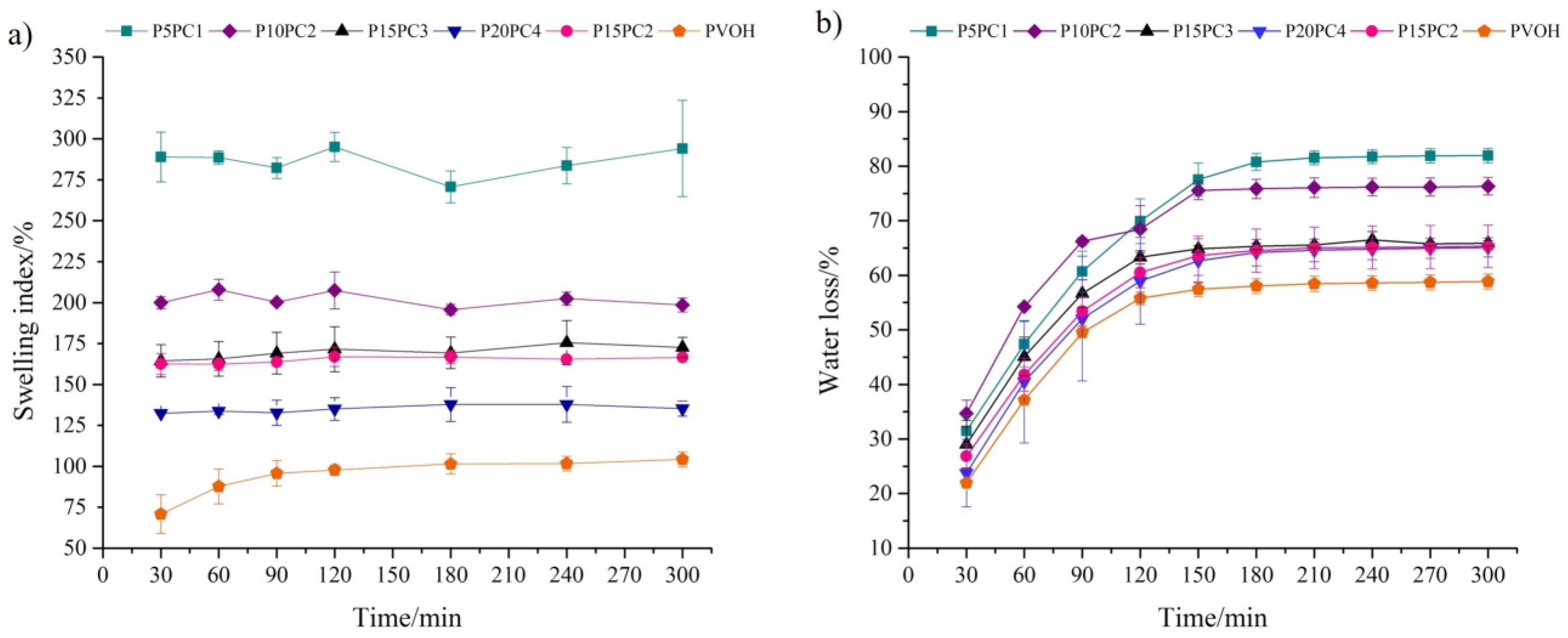
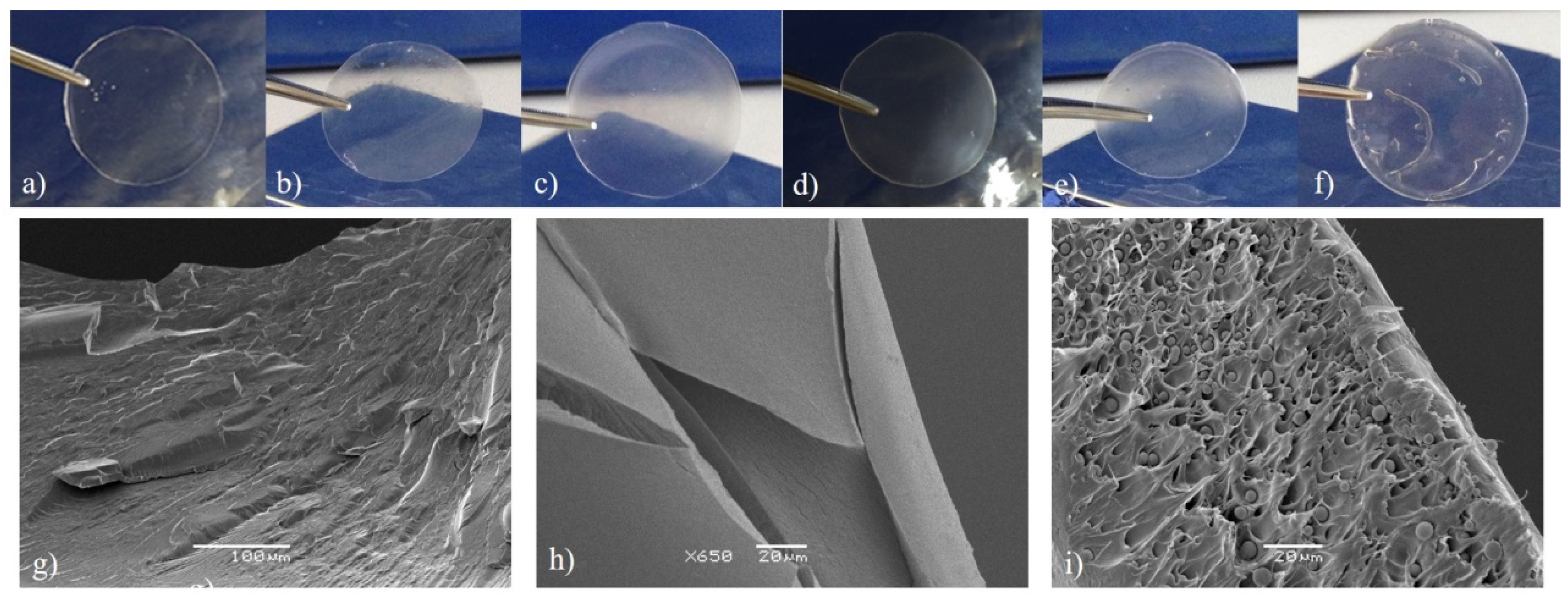
2.1. Physical-Chemical Characterization of the Hydrogel
2.2. Optimization of Hydrogel-Solid Phase Extraction (SPE) Conditions
2.2.1. Effect of pH
2.2.2. Effect of Sample Volume and Flow Rate on the Extraction Efficiency
2.2.3. Optimization of Desorption Volume
2.3. Quantitative Parameters and Analytical Comparison with other Methods
2.4. Analysis of Real Samples
3. Materials and Methods
3.1. Chemicals and Supplies
3.2. Preparation of the Hydrogel Disks
3.3. Physical-Chemical Characterization of the Hydrogel
3.4. Scanning Electron Microscopy (SEM) Analysis
3.5. Hydrogel-SPE Procedure
3.6. Derivatization Step and Gas Chromatography-Tandem Mass Spectrometry (GC-MS/MS) Analysis
3.7. Surface Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghani, M.; Palomino Cabello, C.; Saraji, M.; Manuel Estela, J.; Cerdà, V.; Turnes Palomino, G.; Maya, F. Automated solid-phase extraction of phenolic acids using layered double hydroxide–alumina–polymer disks. J. Sep. Sci. 2018, 41, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M. Before the injection–modern methods of sample preparation for separation techniques. J. Chromatogr. A 1000, 3–27. [Google Scholar] [CrossRef]
- Augusto, F.; Carasek, E.; Silva, R.G.; Rivellino, S.R.; Batista, A.D.; Martendal, E. New sorbents for extraction and microextraction techniques. J. Chromatogr. A 2010, 1217, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Kung, P.-H.; Lee, Y.-D. Preparation of poly(vinyl alcohol)-chondroitin sulfate hydrogel as matrices in tissue engineering. Carbohydr. Polym. 2005, 61, 348–354. [Google Scholar] [CrossRef]
- Childs, A.; Li, H.; Lewittes, D.M.; Dong, B.; Liu, W.; Shu, X.; Sun, C.; Zhang, H.F. Fabricating customized hydrogel contact lens. Sci. Rep. 2016, 6, 34905. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.R.; Munarin, F.; Gentilini, R.; Visai, L.; Granja, P.L.; Tanzi, M.C.; Petrini, P. Injectable pectin hydrogels produced by internal gelation: PH dependence of gelling and rheological properties. Carbohydr. Polym. 2014, 103, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 64, 18–23. [Google Scholar] [CrossRef]
- Basheer, C.; Suresh, V.; Renu, R.; Lee, H.K. Development and application of polymer-coated hollow fiber membrane microextraction to the determination of organochlorine pesticides in water. J. Chromatogr. A 2004, 1033, 213–220. [Google Scholar] [CrossRef]
- Castilhos, N.D.B.; Sampaio, N.M.F.M.; da Silva, B.C.; Riegel-Vidotti, I.C.; Grassi, M.T.; Silva, B.J.G. Physical-chemical characteristics and potential use of a novel alginate/zein hydrogel as the sorption phase for polar organic compounds. Carbohydr. Polym. 2017, 174, 507–516. [Google Scholar] [CrossRef]
- Basheer, C.; Vetrichelvan, M.; Valiyaveettil, S.; Lee, H.K. On-site polymer-coated hollow fiber membrane microextraction and gas chromatography-mass spectrometry of polychlorinated biphenyls and polybrominated diphenyl ethers. J. Chromatogr. A 2007, 1139, 157–164. [Google Scholar] [CrossRef]
- Zare, M.; Ramezani, Z.; Rahbar, N. Development of zirconia nanoparticles-decorated calcium alginate hydrogel fibers for extraction of organophosphorous pesticides from water and juice samples: Facile synthesis and application with elimination of matrix effects. J. Chromatogr. A 1473, 28–37. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Azimi, S.; Sharifi, M.B.A.S. Application of central composite design for methyl red dispersive solid phase extraction based on silver nanocomposite hydrogel: Microwave assisted synthesis. Microchem. J. 2017, 133, 358–369. [Google Scholar] [CrossRef]
- Park, J.W.; PARK, J.S.; Ruckenstein, E.L.I. On the Viscoelastic Properties of Poly (vinyl alcohol) and Chemically Crosslinked Poly (vinyl alcohol). Appl. Ploym. 2001, 82, 1816–1823. [Google Scholar] [CrossRef]
- Lopes, L.C.; Simas-Tosin, F.F.; Cipriani, T.R.; Marchesi, L.F.; Vidotti, M.; Riegel-Vidotti, I.C. Effect of low and high methoxyl citrus pectin on the properties of polypyrrole based electroactive hydrogels. Carbohydr. Polym. 2017, 155, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Albero, B.; Sánchez-Brunete, C.; Miguel, E.; Pérez, R.A.; Tadeo, J.L. Analysis of natural-occurring and synthetic sexual hormones in sludge-amended soils by matrix solid-phase dispersion and isotope dilution gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1283, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Gerald, G.; Margarita, G.; Joerg, S.; Gunnar, S.; Karl-Friedrich, A.; Andreas, R. Chemical and pH sensors based on the swelling behavior of hydrogels. Sens. Actuators B Chem. 2005, 111–112, 555–561. [Google Scholar]
- Bizkarguenaga, E.; Ros, O.; Iparraguirre, A.; Navarro, P.; Vallejo, A.; Usobiaga, A.; Zuloaga, O. Solid-phase extraction combined with large volume injection-programmable temperature vaporization-gas chromatography-mass spectrometry for the multiresidue determination of priority and emerging organic pollutants in wastewater. J. Chromatogr. A 2012, 1247, 104–117. [Google Scholar] [CrossRef]
- Jeannine, E.E.; Mara, M.; Jun, N.; Christopher, N.B. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar]
- Trinh, T.; Harden, N.B.; Coleman, H.M.; Khan, S.J. Simultaneous determination of estrogenic and androgenic hormones in water by isotope dilution gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 1668–1676. [Google Scholar] [CrossRef]
- Rafika, B.S.; Sopheak, N.; Ibtissem, G.-A.; Salma, B.; Maïwen, L.C.; Dalila, B.H.-C.; Malika, T.-A.; Michele, T.; Baghdad, O. Simultaneous Detection of 13 Endocrine Disrupting Chemicals in Water by a Combination of SPE-BSTFA Derivatization and GC-MS in Transboundary Rivers (France-Belgium). Water Air Soil Pollut. 2017, 228, 2. [Google Scholar] [CrossRef]
- Zhang, K.; Fent, K. Determination of two progestin metabolites (17α-hydroxypregnanolone and pregnanediol) and different classes of steroids (androgens, estrogens, corticosteroids, progestins) in rivers and wastewaters by high-performance liquid chromatography-tandem mass spe. Sci. Total Environ. 2018, 610–611, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lis, E.; Kumirska, J.; Stepnowski, P. Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS(SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci. Total Environ. 2015, 538, 402–411. [Google Scholar] [CrossRef]
- Noppe, H.; Verheyden, K.; Gillis, W.; Courtheyn, D.; Vanthemsche, P.; De Brabander, H.F. Multi-analyte approach for the determination of ng L-1levels of steroid hormones in unidentified aqueous samples. Anal. Chim. Acta 2007, 586, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Fernando, F.S.; Igor, C.P.; Cassiana, C.M.; Wilson, F.J. Assessing selected estrogens and xenoestrogens in Brazilian surface waters by liquid chromatography-tandem mass spectrometry. Microchem. J. 2010, 96, 92–98. [Google Scholar]
- Zorica, D.; Svetlana, D.; Ivana, V.B.; Mila, D.L. Determination of sterols and steroid hormones in surface water and wastewater using liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. Microchem. J. 2017, 135, 39–47. [Google Scholar]
- Migowska, N.; Caban, M.; Stepnowski, P.; Kumirska, J. Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography-mass spectrometry and gas chromatography with electron capture detection. Sci. Total Environ. 2012, 441, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Alessandra, H.I.; Rodrigo, A.O.; Luana, O.M.; Jorge, D.C.P.; Júlio, C.R.D.A. Occurrence of Pharmaceutical Products, Female Sex Hormones and Caffeine in a Subtropical Region in Brazil. Clean (Weinh) 2017, 45, 334. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Analytes | Concentration Evaluated/µg L−1 | Intra-Assay Precision (n = 3) | Concentration Evaluated/µg L−1 | Inter-Assay Precision (n = 3) | ||||
|---|---|---|---|---|---|---|---|---|
| Measured Conc./µg L−1 | RSD/% | Accuracy/% | Measured Conc./µg L−1 | RSD/% | Accuracy/% | |||
| E1 | 40.0 | 38.6 ± 0.21 | 0.5 | 96.7 | 50.0 | 50.3 ± 2.9 | 5.7 | 100.5 |
| 80.0 | 71.8 ± 2.9 | 4.1 | 89.7 | 100.0 | 102.6 ± 1.9 | 1.9 | 102.6 | |
| E2 | 40.0 | 33.1 ± 1.5 | 1.9 | 82.6 | 50.0 | 50.5 ± 3.1 | 6.2 | 100.9 |
| 80.0 | 63.9 ± 1.5 | 2.4 | 80.0 | 100.0 | 99.8 ± 8.29 | 8.3 | 99.8 | |
| TES | 40.0 | 38.1 ± 4.0 | 10.4 | 95.4 | 50.0 | 50.3 ± 3.0 | 6.0 | 100.6 |
| 80.0 | 72.8 ± 5.2 | 7.2 | 91.0 | 100.0 | 98.7± 3.8 | 3.8 | 98.7 | |
| EE2 | 40.0 | 37.6 ± 2.0 | 5.4 | 94.0 | 50.0 | 46.9 ± 2.8 | 6.0 | 93.7 |
| 80.0 | 85.1 ± 7.6 | 8.9 | 106.3 | 100.0 | 100.8 ± 4.8 | 4.7 | 100.8 | |
| PRO | 40.0 | 35.6 ± 4.1 | 11.5 | 88.9 | 50.0 | 49.1 ± 10.9 | 22.2 | 98.1 |
| 80.0 | 83.7 ± 10.3 | 12.3 | 104.7 | 100.0 | 102.4 ± 6.8 | 6.6 | 102.4 | |
| E3 | 40.0 | 40.4 ± 3.0 | 7.3 | 101.0 | 50.0 | 49.9 ± 1.7 | 3.4 | 99.7 |
| 80.0 | 85.0 ± 6.5 | 7.6 | 106.2 | 100.0 | 108,2 ± 8.6 | 7.9 | 108.2 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. F. M. Sampaio, N.; D. B. Castilhos, N.; C. da Silva, B.; C. Riegel-Vidotti, I.; J. G. Silva, B. Evaluation of Polyvinyl Alcohol/Pectin-Based Hydrogel Disks as Extraction Phase for Determination of Steroidal Hormones in Aqueous Samples by GC-MS/MS. Molecules 2019, 24, 40. https://doi.org/10.3390/molecules24010040
M. F. M. Sampaio N, D. B. Castilhos N, C. da Silva B, C. Riegel-Vidotti I, J. G. Silva B. Evaluation of Polyvinyl Alcohol/Pectin-Based Hydrogel Disks as Extraction Phase for Determination of Steroidal Hormones in Aqueous Samples by GC-MS/MS. Molecules. 2019; 24(1):40. https://doi.org/10.3390/molecules24010040
Chicago/Turabian StyleM. F. M. Sampaio, Naiara, Natara D. B. Castilhos, Bruno C. da Silva, Izabel C. Riegel-Vidotti, and Bruno J. G. Silva. 2019. "Evaluation of Polyvinyl Alcohol/Pectin-Based Hydrogel Disks as Extraction Phase for Determination of Steroidal Hormones in Aqueous Samples by GC-MS/MS" Molecules 24, no. 1: 40. https://doi.org/10.3390/molecules24010040