Lignans from Bursera fagaroides Affect In Vivo Cell Behavior by Disturbing the Tubulin Cytoskeleton in Zebrafish Embryos
Abstract
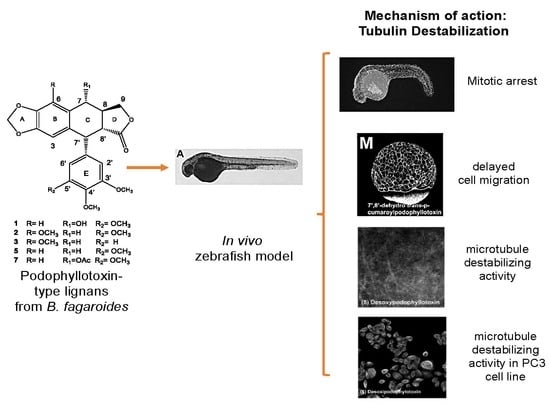
:1. Introduction
2. Results and Discussion
2.1. In Vivo Analysis of Mitotic Arrest in Zebrafish Embryos
2.2. Characterization of Compounds that Affect Cell Migration in the Zebrafish Model
2.3. In Vivo and In Vitro Analysis of Compounds that Affect Microtubules
3. Materials and Methods
3.1. Plant Material, Extraction, and Isolation
3.2. Fish Maintenance and Strains
3.3. Chemical Treatment of Zebrafish Embryos
3.4. Immunofluorescense and Immunohistochemistry
3.5. Fluorescence Microscopy
3.6. Confocal Laser Scanning Microscopy
3.7. Immunofluorescence of α-Tubulin in PC3 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayres, D.C.; Loike, J.D. Lignans Chemical, Biological and Clinical Properties; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Teponno, R.B.; Kusari, S.M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilkington, L. Lignans: A Chemometric Analysis. Molecules 2018, 23, 1666. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.J.; Wang, Y.J.; Liang, Y.C.; Jeng, J.H.; Lee, W.S.; Lin, J.K.; Chen, C.H.; Liu, I.C.; Ho, Y.S. Microtubule damaging agents induce apoptosis in HL 60 cells and G2/M cell cycle arrest in HT 29 cells. Toxicology 2002, 175, 123–142. [Google Scholar] [CrossRef]
- Gordaliza, M.; Garcia, P.A.; del Corral, J.M.; Castro, M.A.; Gomez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Antunez-Mojica, M.; Rodríguez-Salarichs, J.; Redondo-Horcajo, M.; León, A.; Barasoain, I.; Canales, A.; Cañada, F.J.; Jiménez-Barbero, J.; Alvarez, L.; Díaz, J.F. Structural and Biochemical Characterization of the Interaction of Tubulin with Potent Natural Analogues of Podophyllotoxin. J. Nat. Prod. 2016, 79, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Hande, K.R. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Rojas-Sepulveda, A.M.; Mendieta-Serrano, M.; Mojica, M.Y.; Salas-Vidal, E.; Marquina, S.; Villarreal, M.L.; Puebla, A.M.; Delgado, J.I.; Alvarez, L. Cytotoxic Podophyllotoxin Type-Lignans from the Steam Bark of Bursera fagaroides var. fagaroides. Molecules 2012, 17, 9506–9519. [Google Scholar] [CrossRef] [Green Version]
- Antunez-Mojica, M.; León, A.; Rojas-Sepúlveda, A.M.; Marquina, S.; Mendieta-Serrano, M.; Salas-Vidal, E.; Villareal, M.L.; Alvarez, L. Aryldihydronaphthalene-type lignans from Bursera fagaroides var. fagaroides and their antimitotic mechanism of action. RSC Adv. 2016, 6, 4950–4959. [Google Scholar] [CrossRef]
- Peña-Morán, O.; Villareal, M.L.; Álvarez, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, Post-Treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera fagaroides var. fagaroides on Breast Cancer Cell Lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, F.; Puebla-Pérez, A.M.; González-Pozos, S.; Hernández-Hernández, J.M.; Pérez-Rangel, A.; Alvarez, L.; Tapia-Pastrana, G.; Castillo-Romero, A. Antigiardial Activity of Podophyllotoxin-Type Lignans from Bursera fagaroides var. fagaroides. Molecules 2017, 22, 799. [Google Scholar] [CrossRef]
- Hendzel, M.J.; Wei, Y.; Mancini, M.A.; Van Hooser, A.; Brinkley, B.R.; Bazett-Jones, D.P.; Allis, C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997, 106, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Mendieta-Serrano, M.A.; Schnabel, D.; Lomeli, H.; Salas-Vidal, E. Cell proliferation patterns in early zebrafish development. Anat. Rec. 2013, 296, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Saka, Y.; Smith, J.C. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev. Biol. 2001, 229, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kendrick, C.; Julich, D.; Holley, S.A. Cell cycle progression is required for zebrafish somite morphogenesis but not segmentation clock function. Development 2008, 135, 2065–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphey, R.D.; Stern, H.M.; Straub, C.T.; Zon, L.I. A chemical genetic screen for cell cycle inhibitors in zebrafish embryos. Chem. Biol. Drug Des. 2006, 68, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Urbani, L.; Sherwood, S.W.; Schimke, R.T. Dissociation of nuclear and cytoplasmic cell cycle progression by drugs employed in cell synchronization. Exp. Cell Res. 1995, 219, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, R.; Zhang, J.; Rivera-Bennetts, A.K.; Yager, T.D. Activation of the metaphase checkpoint and an apoptosis programme in the early zebrafish embryo, by treatment with the spindle-destabilising agent nocodazole. Zygote (Cambridge, England) 1997, 5, 329–350. [Google Scholar] [CrossRef]
- Solnica-Krezel, L.; Driever, W. Microtubule arrays of the zebrafish yolk cell: Organization and function during epiboly. Development (Cambridge, England) 1994, 120, 2443–2455. [Google Scholar]
- Strahle, U.; Jesuthasan, S. Ultraviolet irradiation impairs epiboly in zebrafish embryos: Evidence for a microtubule-dependent mechanism of epiboly. Development (Cambridge, England) 1993, 119, 909–919. [Google Scholar]
- Moon, H.S.; Jacobson, E.M.; Khersonsky, S.M.; Luzung, M.R.; Walsh, D.P.; Xiong, W.; Lee, J.W.; Parikh, P.B.; Lam, J.C.; Kang, T.W.; et al. A novel microtubule destabilizing entity from orthogonal synthesis of triazine library and zebrafish embryo screening. J. Am. Chem. Soc. 2002, 124, 11608–11609. [Google Scholar] [CrossRef]
- Jesuthasan, S.; Stahle, U. Dynamic microtubules and specification of the zebrafish embryonic axis. Curr. Biol. 1997, 7, 31–42. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Strahle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio); Institute of Neuroscience, University of Oregon: Eugene, OR, USA, 2000. [Google Scholar]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Alejandre-García, I.; Álvarez, L.; Cardoso-Taketa, A.; González-Maya, L.; Antúnez, M.; Salas-Vidal, E.; Díaz, J.F.; Marquina-Bahena, S.; Villarreal, M.A. Cytotoxic Activity and Chemical Composition of the Root Extract from the Mexican Species Linum scabrellum: Mechanism of Action of the Active Compound 6-Methoxypodophyllotoxin. Evid. Based Complement. Alternat. Med. 2015, 2015, 298463. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antúnez-Mojica, M.; Rojas-Sepúlveda, A.M.; Mendieta-Serrano, M.A.; Gonzalez-Maya, L.; Marquina, S.; Salas-Vidal, E.; Alvarez, L. Lignans from Bursera fagaroides Affect In Vivo Cell Behavior by Disturbing the Tubulin Cytoskeleton in Zebrafish Embryos. Molecules 2019, 24, 8. https://doi.org/10.3390/molecules24010008
Antúnez-Mojica M, Rojas-Sepúlveda AM, Mendieta-Serrano MA, Gonzalez-Maya L, Marquina S, Salas-Vidal E, Alvarez L. Lignans from Bursera fagaroides Affect In Vivo Cell Behavior by Disturbing the Tubulin Cytoskeleton in Zebrafish Embryos. Molecules. 2019; 24(1):8. https://doi.org/10.3390/molecules24010008
Chicago/Turabian StyleAntúnez-Mojica, Mayra, Andrés M. Rojas-Sepúlveda, Mario A. Mendieta-Serrano, Leticia Gonzalez-Maya, Silvia Marquina, Enrique Salas-Vidal, and Laura Alvarez. 2019. "Lignans from Bursera fagaroides Affect In Vivo Cell Behavior by Disturbing the Tubulin Cytoskeleton in Zebrafish Embryos" Molecules 24, no. 1: 8. https://doi.org/10.3390/molecules24010008