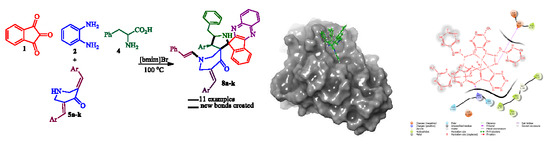
Domino Multicomponent Approach for the Synthesis of Functionalized Spiro-Indeno[1,2-b]quinoxaline Heterocyclic Hybrids and Their Antimicrobial Activity, Synergistic Effect and Molecular Docking Simulation
Abstract
:1. Introduction
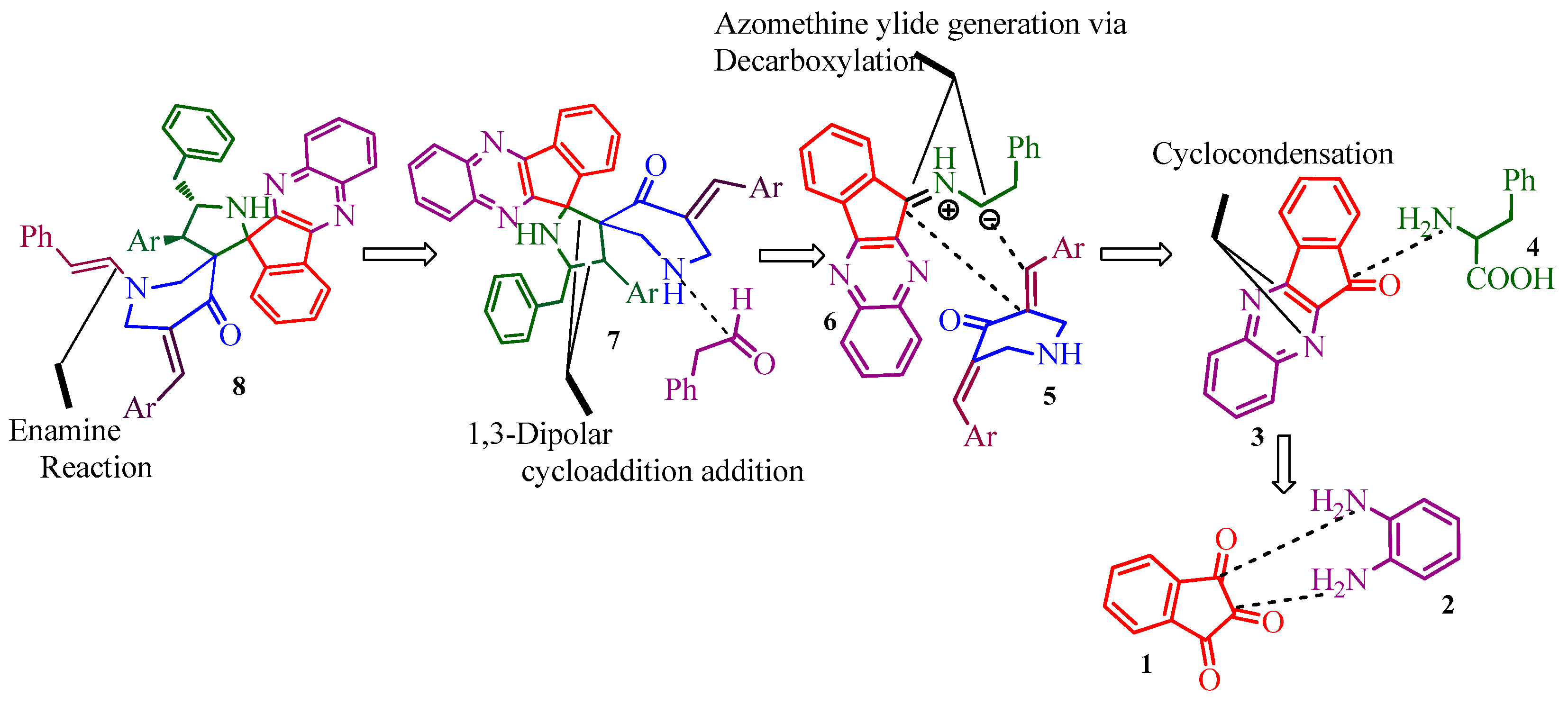
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antibacterial Activities
2.2.2. Synergistic Activity
2.2.3. Antifungal Activity
2.3. Docking Simulation
3. Material and Methods
General Procedure for Synthesis of Dispiropyrrolidine Heterocyclic hybrids, 8a–k
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Peng, X.M.; Damu, G.L.V.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Tice, C.M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert. Opin. Drug. Discov. 2016, 11, 831–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Yu, D.Q.; Liu, H.M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Kathirvelan, D.; Haribabu, J.; Reddy, B.S.R.; Balachandran, C.; Duraipandiyan, V. Facile and diastereoselective synthesis of 3,2′-spiropyrrolidine-oxindoles derivatives, their molecular docking and antiproliferative activities. Bioorg. Med. Chem. Lett. 2015, 25, 389–399. [Google Scholar] [CrossRef]
- Arun, Y.; Saranraj, K.; Balachandran, C.; Perumal, P.T. Novel spirooxindole-pyrrolidine compounds: synthesis, anticancer and molecular docking studies. Eur. J. Med. Chem. 2014, 74, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Perumal, S.; Menendez, J.C.; Yogeeswari, P.; Sriram, D. Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med. Chem. Commun. 2011, 2, 626–630. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Ramakrishna, S.; Reddy, K.G.; Nagaraju, D.; Reddy, Y.N. A facile synthesis, anti-inflammatory and analgesic activity of isoxazolyl-2,3-dihydrospiro[benzo[f]isoindole-1,3′-indoline]-2′,4,9-triones. Bioorg. Med. Chem. Lett. 2013, 23, 3954–3958. [Google Scholar] [CrossRef]
- Bhaskar, G.; Arun, Y.; Balachandran, C.; Saikumar, C.; Perumal, P.T. Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur. J. Med. Chem. 2012, 51, 79–91. [Google Scholar] [CrossRef]
- Kia, Y.; Osman, H.; Suresh Kumar, R.; Basiri, A.; Murugaiyah, V. Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Suresh Kumar, R. A facile chemo-, regio- and stereoselective synthesis and cholinesterase inhibitory activity of spirooxindole-pyrrolizine-piperidine hybrids. Bioorg. Med. Chem. Lett. 2013, 23, 2979–2983. [Google Scholar] [CrossRef]
- Santiago-Vazquez, Y.; Das, S.; Das, U.; Robles-Escajeda, E.; Ortega, N.M.; Lema, C.; Varela-Ramírez, A.; Aguilera, R.J.; Balzarini, J.; Clercq, E.D.; et al. Novel 3,5-bis(arylidene)-4-oxo-1-piperidinyl dimers: structure-activity relationships and potent antileukemic and antilymphoma cytotoxicity. Eur. J. Med. Chem. 2014, 77, 315–322. [Google Scholar] [CrossRef]
- Harini, S.T.; Kumar, H.V.; Rangaswamy, J.; Naik, N. Synthesis, antioxidant and antimicrobial activity of novel vanillin derived piperidin-4-one oxime esters: preponderant role of the phenyl ester substituents on the piperidin-4-one oxime core. Bioorg. Med. Chem. Lett. 2012, 22, 7588–7592. [Google Scholar] [CrossRef]
- Suresh Kumar, R.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Alshahrani, W.S.; Kotresha, D.; Altaf, M.; Azam, M.; Menendez, J.C. Highly functionalized pyrrolidine analogues: stereoselective synthesis and caspase-dependent apoptotic activity. RSC Adv. 2018, 8, 41226–41236. [Google Scholar] [CrossRef] [Green Version]
- Suresh Kumar, R.; Rajesh, S.M.; Banerjee, D.; Yogeeswari, P.; Sriram, D. Novel three-component domino reactions of ketones, isatin and amino acids: synthesis and discovery of antimycobacterial activity of highly functionalised novel dispiropyrrolidines. Eur. J. Med. Chem. 2010, 45, 411–422. [Google Scholar] [CrossRef]
- Arumugam, N.; Suresh Kumar, R.; Almansour, A.I.; Altaf, M.; Padmanaban, R.; Suresh babu, P.; Angamuthu, G.; Kotresha, D.; Manohar, T.S.; Venketesh, S. Spiropyrrolidine/spiroindolizino[6,7-b]indole heterocyclic hybrids: Stereoselective synthesis, cholinesterase inhibitory activity and their molecular docking study. Bioorg. Chem. 2018, 79, 64–71. [Google Scholar] [CrossRef]
- Arumugam, N.; Periyasami, G.; Raghunathan, R.; Kamalraj, S.; Muthumary, J. Synthesis and antimicrobial activity of highly functionalised novel β-lactam grafted spiropyrrolidines and pyrrolizidines. Eur. J. Med. Chem. 2011, 46, 600–607. [Google Scholar] [CrossRef]
- Arumugam, N.; Raghunathan, R.; Shanmugaiah, V.; Mathivanan, N. Synthesis of novel beta-lactam fused spiroisoxazolidine chromanones and tetralones as potent antimicrobial agent for human and plant pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 3698–3702. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Padmani-layam, M.P.; Puthucode, R.N.; Nazarali, A.J.; Motaganahalli, N.L.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Kraatz, H.B.; Prisciak, J.S.; et al. A conformational and structure-activity relationship study of cytotoxic 3,5-bis(arylidene)-4-piperidones and related N-acryloyl analogues. J. Med. Chem. 2001, 44, 586–593. [Google Scholar] [CrossRef]
- Almansour, A.I.; Arumugam, N.; Suresh Kumar, R.; Subbarayan, P.V.; Alshatwi, A.A.; Ghabbour, H.A. Anticancer Agents. U.S. Patent 9,486,444 B1, 8 November 2016. [Google Scholar]
- Almansour, A.I.; Arumugam, N.; Suresh Kumar, R.; Subbarayan, P.V.; Alshatwi, A.A.; Athinarayanan, J. Anticancer Agents. U.S. Patent 9,873,699B1, 23 Janauary 2018. [Google Scholar]
- Ping, Q.; Yanling, D.; Haibo, M.; Soloshonok, V.A.; Jianlin, H.; Yi, P. Ni-catalyzed asymmetric decarboxylative Mannich reaction for the synthesis of β-trifluoromethyl-β-amino ketones. RSC Adv. 2015, 5, 26811–26814. [Google Scholar]
- Wu, L.; Xie, C.; Mei, H.; Dai, Y.; Han, J.; Soloshonok, V.A.; Pan, Y. Synthesis of Trifluoromethyl-Containing Vicinal Diamines by Asymmetric Decarboxylative Mannich Addition Reactions. J. Org. Chem. 2015, 80, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Govindasami, P.; Al-thamili, D.M.; Krishnamoorthy, R.; Periasamy, V.S.; Alshatwi, A.A.; Mahalingam, S.M.; Thangamani, S.; et al. Multicomponent Domino Synthesis, Anticancer Activity and Molecular Modeling Simulation of Complex Dispirooxindolopyrrolidines. Molecules 2018, 23, 1094. [Google Scholar] [CrossRef]
- Arumugam, N.; Abdulrahman, I.A.; Suresh Kumar, R.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A.; Periyasami, G.; Altaf, M.; Menéndez, J.C. Regio- and diastereoselective synthesis of anticancer spirooxindoles derived from tryptophan and histidine via three-component 1,3-dipolar cycloadditions in an ionic liquid. Tetrahedron 2018, 74, 5358–5366. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 6, 1295–1301. [Google Scholar] [CrossRef]
- Kuhn, B.; Guba, W.; Hert, J.; Banner, D.; Bissantz, C.; Ceccarelli, S.; Haap, W.; Korner, M.; Kuglstatter, A.; Lerner, C.; et al. A Real-World Perspective on Molecular Design. J. Med. Chem. 2016, 59, 4087–4102. [Google Scholar] [CrossRef]
- Premnath, D.; Enoch, I.V.; Selvakumar, P.M.; Indiraleka, M.; Vennila, J.J. Design, Synthesis, Spectral Analysis, In Vitro Anticancer Evaluation and Molecular Docking Studies of Some Fluorescent 4-Amino-2, 3-Dimethyl-1-Phenyl-3-Pyrazolin-5-One, Ampyrone Derivatives. Interdiscip. Sci. 2017, 9, 130–139. [Google Scholar] [CrossRef]
- Takeda, K.; Miyatake, H.; Yokota, N.; Matsuyama, S.I.; Tokuda, H.; Miki, K. Crystal structure of bacterial lipoprotein localization factor, LoIB. EMBO J. 2003, 22, 3199–3209. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |









| Entry | Solvents | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | methanol | 3 | 49 |
| 2 | ethanol | 3 | 45 |
| 3 | acetonitrile | 3 | 42 |
| 4 | 1,4-dioxane | 3 | 40 |
| 5 | 1,4-dioxane: Methanol (1:1V/V) | 3 | 46 |
| 6 | [bmim]Br | 1 | 64 |
| Bacterial Pathogens | MIC µg/mL | |
|---|---|---|
| Compound 8h | Streptomycin | |
| Gram-positive bacterial pathogens | ||
| Staphylococcus aureus MTCC 96 | 125.00 | 10.0 |
| Staphylococcus epidermidis MTCC 3615 | 31.25 | 5.0 |
| Bacillus subtilis MTCC 441 | 31.25 | 5.0 |
| Gram-negative bacterial pathogens | ||
| Escherichia coli ATCC 25922 | 62.50 | 5.0 |
| Pseudomonas aeruginosa ATCC 27584 | 250.00 | 10.0 |
| Klebsiella pneumoniae MTCC 109 | 15.60 | 5.0 |
| Proteus vulgaris ATCC 8427 | 62.50 | 10.0 |
| Proteus mirabilis ATCC 7002 | 125.00 | 10.0 |
| Salmonella typhi ATCC 19430 | 15.60 | 5.0 |
| Salmonella paratyphi MTCC 735 | 31.25 | 5.0 |
| Compound and Antibiotics Combinations (µg/mL) | MICa | MICb | FIC | FICI |
|---|---|---|---|---|
| Compound 8h-Streptomycin | 0.5 | |||
| Compound 8h | 62.50 | 15.60 | 0.25 | |
| Streptomycin | 10.0 | 2.5 | 0.25 | |
| Compound 8h-Tetracycline | 1.0 | |||
| Compound 8h | 62.50 | 31.25 | 0.25 | |
| Tetracycline | 15.0 | 15.0 | 0.5 | |
| Compound 8h-Vancomycin | 0.75 | |||
| Compound 8h | 62.50 | 31.25 | 0.5 | |
| Vancomycin | 30.0 | 7.5 | 0.25 |
| Molecular Docking | ||||
|---|---|---|---|---|
| Compound | Glide Score (Kcal/mol) | Emodel Score | Glide Energy | XP Hydrogen Bond |
| 8h | −4.376 | −48.79 | −39.576 | 2 (ASP96, ARG115) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansour, A.I.; Arumugam, N.; Suresh Kumar, R.; Al-thamili, D.M.; Periyasami, G.; Ponmurugan, K.; Al-Dhabi, N.A.; Perumal, K.; Premnath, D. Domino Multicomponent Approach for the Synthesis of Functionalized Spiro-Indeno[1,2-b]quinoxaline Heterocyclic Hybrids and Their Antimicrobial Activity, Synergistic Effect and Molecular Docking Simulation. Molecules 2019, 24, 1962. https://doi.org/10.3390/molecules24101962
Almansour AI, Arumugam N, Suresh Kumar R, Al-thamili DM, Periyasami G, Ponmurugan K, Al-Dhabi NA, Perumal K, Premnath D. Domino Multicomponent Approach for the Synthesis of Functionalized Spiro-Indeno[1,2-b]quinoxaline Heterocyclic Hybrids and Their Antimicrobial Activity, Synergistic Effect and Molecular Docking Simulation. Molecules. 2019; 24(10):1962. https://doi.org/10.3390/molecules24101962
Chicago/Turabian StyleAlmansour, Abdulrahman I., Natarajan Arumugam, Raju Suresh Kumar, Dhaifallah M. Al-thamili, Govindasami Periyasami, Karuppiah Ponmurugan, Naif Abdullah Al-Dhabi, Karthikeyan Perumal, and Dhanaraj Premnath. 2019. "Domino Multicomponent Approach for the Synthesis of Functionalized Spiro-Indeno[1,2-b]quinoxaline Heterocyclic Hybrids and Their Antimicrobial Activity, Synergistic Effect and Molecular Docking Simulation" Molecules 24, no. 10: 1962. https://doi.org/10.3390/molecules24101962