The Role Ionic Liquid [BMIM][PF6] in One-Pot Synthesis of Tetrahydropyran Rings through Tandem Barbier–Prins Reaction
Abstract
:1. Introduction
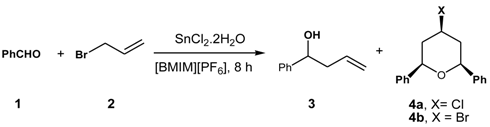
2. Results and Discussion
3. Experimental
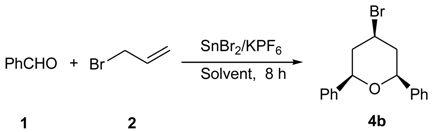
3.1. Synthesis of 2,4,6-Trisubstituted Tetrahydropyran Compounds (General Procedure)
3.2. Characterization Data [32a]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolaou, k.C.; Theodorakis, E.A.; Rutjes, F.P.J.T.; Tiebes, J.; Sato, M.; Untersteller, E.; Xiao, X.-Y.; Total Synthesis of Brevetoxin, B. 1. CDEFG Framework. J. Am. Chem. Soc. 1995, 117, 1171–1172. [Google Scholar] [CrossRef]
- Vasconcellos, M.L.A.A.; Miranda, L.S.M. A reação de ciclização de Prins: uma estratégia eficiente para síntese estereosseletiva de anéis tetraidropirânicos substituídos. Quím. Nova 2006, 29, 834–839. [Google Scholar] [CrossRef]
- Umamatheswari, S.; Balaji, B.; Ramanathan, M.; Kabilan, S. Synthesis, antimicrobial evaluation and QSAR studies of novel piperidin-4-yl-5-spiro-thiadiazoline derivatives. Eur J. of Med. Chem. 2011, 46, 1415–1429. [Google Scholar] [CrossRef]
- Masuda, K.; Nishiwaki, H.; Akiyama, K.; Yamauchi, S.; Maruyama, M.; Sugahara, T.; Kishida, T. Antifungal Activity of Morinol B Derivatives of Tetrahydropyran Sesquilignan. Bio. Biotec. Biochem. 2010, 74, 2071–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.P.L.; Dantas, B.B.; Martins, G.V.F.; de Araújo, D.A.M.; Vasconcellos, M.L.A.A. Synthesis and Anticancer Activities of Novel Guanylhydrazone and Aminoguanidine Tetrahydropyran Derivatives. Molecules 2016, 21, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Anderson, D.D. Tetrahydrofuran, tetrahydropyran, triazoles and related heterocyclic derivatives as HIV protease inhibitors. Future Med. Chem. 2011, 3, 1181–1197. [Google Scholar] [CrossRef] [Green Version]
- Miranda, L.S.M.; Marinho, B.G.; Leitão, S.G.; Matheus, M.E.; Fernandes, P.D.; Vasconcellos, M.L.A.A. (±)-cis-(6-Ethyl-tetrahydropyran-2-yl)-formic acid: a novel substance with antinociceptive properties. Bioorg. Med. Chem. 2004, 14, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bhardwaj, A.; Kaur, S.; Kumar, S. Design, synthesis and evaluation of tetrahydropyran based COX-1/-2 inhibitors. Eur. J. Med. Chem. 2009, 44, 1278–1287. [Google Scholar] [CrossRef]
- Nitta, A.; Fujii, H.; Sakami, S.; Nishimura, Y.; Ohyama, T.; Satoh, M.; Nakaki, J.; Satoh, S.; Inada, C.; Kozono, H.; et al. (3R)-3-Amino-4-(2,4,5-trifluorophenyl)-N-{4-[6-(2-methoxyethoxy)benzothiazol-2-yl]tetrahydropyran-4-yl}butanamide as a potent dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg. Med. Chem. Lett. 2008, 18, 5435–5438. [Google Scholar] [CrossRef] [PubMed]
- Mulzer, J.; Meyer, F.; Buschmann, J.; Luger, P. Asymmetric synthesis of the C-26-C-32 tetrahydropyran — moiety of Swinholide A by hetero-Diels-Alder reaction. Tetrahedron Lett. 1995, 36, 3503–3506. [Google Scholar] [CrossRef]
- Bolitt, V.; Mioskowski, C.; Bhatt, R.K.; Falck, J.R. Synthesis of (S)-(+)-mevalonolactone and mevalonate analogs. J. Org. Chem. 1991, 56, 4238–4240. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Aprile, C.; Riela, S.; Noto, R. Synthesis of 2,4,6-trisubstituted tetrahydropyrans via 6-exo selenoetherification of unsaturated alcohols. Tetrahedron Lett. 2001, 42, 2213–2215. [Google Scholar] [CrossRef]
- Mukai, C.; Ikeda, Y.; Sugimoto, Y.-I.; Hanaoka, M. Regioselective and stereospecific formation of 2-ethynyl-3-hydroxytetrahydropyran derivatives via 6-endo ring closure. Tetrahedron Lett. 1994, 35, 2179–2182. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Frederick, M.O.; Aversa, R.J. The Continuing Saga of the Marine Polyether Biotoxins. Angew. Chem. Int. Ed. 2008, 47, 7182–7225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, P.A.; Cui, J.; Santosh, J.G.; Polosukhin, A.; Zhang, H.-R. Enantioselective Total Synthesis of the Potent Antitumor Agent (−)-Mucocin Using a Temporary Silicon-Tethered Ring-Closing Metathesis Cross-Coupling Reaction. J. Am. Chem. Soc. 2003, 125, 14702–14703. [Google Scholar] [CrossRef]
- Domon, D.; Fujiwara, K.; Ohtaniuchi, Y.; Takezawa, A.; Takeda, S.; Kawasaki, H.; Murai, A.; Kawai, H.; Suzuki, T. Synthesis of the C42–C52 part of ciguatoxin CTX3C. Tetrahedron Lett. 2005, 46, 8279–8283. [Google Scholar] [CrossRef]
- Takahashi, S.; Kubota, A.; Nakata, T. Stereoselective Total Synthesis of Mucocin, an Antitumor Agent. Angew. Chem. Int. Ed. 2002, 41, 4751–4754. [Google Scholar] [CrossRef]
- Bravo, F.; McDonald, F.E.; Neiwert, W.A.; Do, B.; Hardcastle, I.K. Biomimetic Synthesis of Fused Polypyrans: Oxacyclization Stereo- and Regioselectivity Is a Function of the Nucleophile. Org. Lett. 2003, 5, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Fujinami, K.; Maitoko, Y.; Ueda, K.; Kakiuchi, K. Lewis Acid-Promoted Cyclization Reactions of Alkenyl Ethenetricarboxylates: Stereoselective Synthesis of 2-Oxotetrahydrofurans and 2-Oxopyrrolidines. J. Org. Chem. 2013, 78, 8405–8416. [Google Scholar] [CrossRef]
- Yadav, V.K.; Verma, A.K.; Kumar, P.; Hulika, V. 2-Arylcyclopropylmethanol as a substitute for homoallyl aryl alcohol in the construction of cis-2,6-disubstituted tetrahydropyran: synthesis of (±)-centrolobine. Chem. Commun. 2014, 50, 15457–15460. [Google Scholar] [CrossRef]
- Spivey, A.C.; Laraia, L.; Bayly, A.R.; Rzepa, H.S.; White, A.J.P. Stereoselective Synthesis of cis- and trans-2,3-Disubstituted Tetrahydrofurans via Oxonium−Prins Cyclization: Access to the Cordigol Ring System. Org. Lett. 2010, 12, 900–903. [Google Scholar] [CrossRef]
- Lee, C.H.A.; Loh, T.P. Suppression of epimerization due to selectivity leakage: an application towards the total synthesis of (−)-centrolobine. Tetrahedron Lett. 2006, 47, 1641–1644. [Google Scholar] [CrossRef]
- Dam, J.H.; Fristrup, P.; Madsen, R. Combined Experimental and Theoretical Mechanistic Investigation of the Barbier Allylation in Aqueous Media. J. Org. Chem. 2008, 73, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Olier, C.; Kaafarani, M.; Gastaldi, S.; Bertrand, M.P. Synthesis of tetrahydropyrans and related heterocycles via prins cyclization; extension to aza-prins cyclization. Tetrahedron 2010, 66, 413–445. [Google Scholar] [CrossRef]
- Yi, X.H.; Haberman, J.X.; Li, C.J. Metal-Mediated Barbier-Type Carbonyl Allylation Under Solvent-Free Conditions. Synthetic Comm. 1998, 28, 2999–3009. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A General 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, X.; Liu, L.; Chen, Y.-J. Direct synthesis of tetrahydropyrans via one-pot Babier–Prins cyclization of allylbromide with carbonyl compounds promoted by RTILs BPyX/SnX′2 or BBIMBr/SnBr2. Tetrahedron 2006, 62, 7113–7120. [Google Scholar] [CrossRef]
- Tang, L.; Ding, L.; Chang, W.X.; Li, J. SnCl2-mediated carbonyl allylation of aldehydes and ketones in ionic liquid. Tetrahedron Lett. 2006, 47, 303–306. [Google Scholar] [CrossRef]
- Gordon, C.M.; McCluskey, A. Ionic liquids: A convenient solvent for environmentally friendly allylation reactions with tetraallylstannane. Chem. Commun. 1999, 1431–1432. [Google Scholar] [CrossRef]
- Cassol, C.C.; Ebeling, G.; Ferreira, B.; Dupont, J.A. A Simple and Practical Method for the Preparation and Purity Determination of Halide-Free Imidazolium Ionic Liquids. Adv. Synth. Catal. 2006, 348, 243–248. [Google Scholar] [CrossRef]
- Houllemare, D.; Outurquin, F.; Paulmier, C. Synthesis of homoallylic (but-3-enylic) alcohols from aldehydes with allylic chlorides, tin(II) chloride and potassium iodide in water. J. Chem. Soc. Perkin Trans. 1 1997, 11, 1629–1632. [Google Scholar] [CrossRef]
- Slaton, R.; Petrone, A.; Manchanayakage, R. Tin mediated Barbier type allylation in ionic liquids. Tetrahedron Lett. 2011, 52, 5073–5076. [Google Scholar] [CrossRef]
- Silva, F.P.L.; Sabino, J.R.; Martins, F.T.; Vasconcellos, M.L.A.A. Diastereoselective syntheses via Prins cyclization, crystal structures determination and theoretical studies of cis-2,6-diphenyl-4-hydroxytetrahydropyran and analogues. J. Mol. Struct. 2013, 1036, 478–487. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


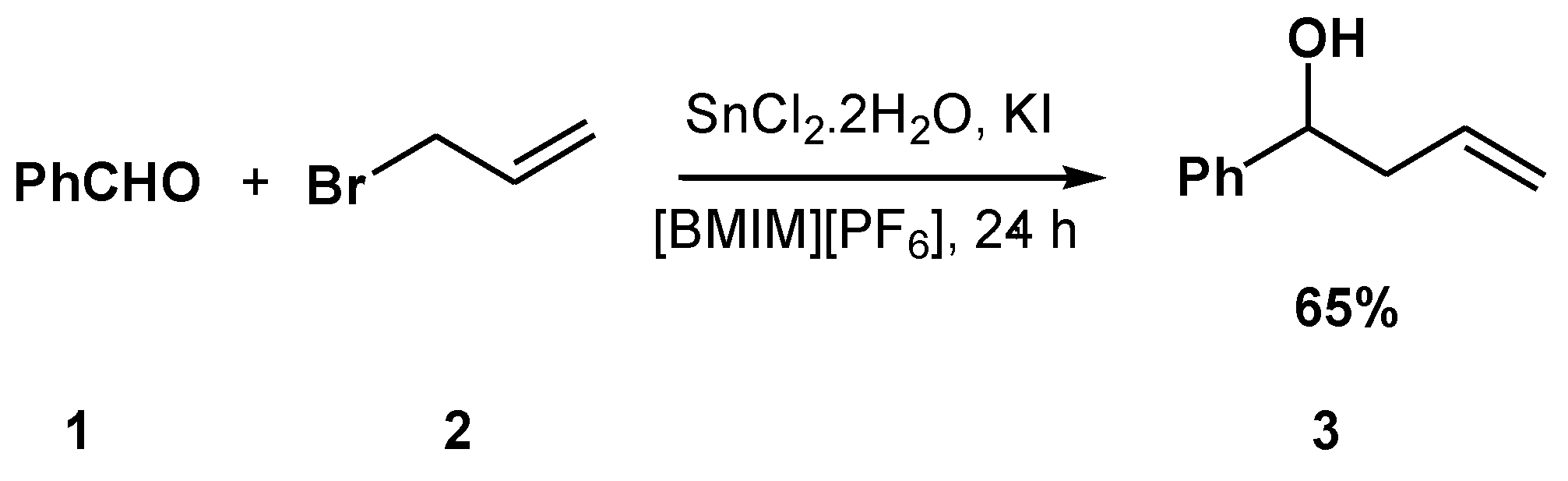


| Entry a | 1:2 Ratio | Conversion (%) b | Ratio b (3:4a:4b)% |
|---|---|---|---|
| 1 | 1:1.5 | 12 | 0:92:8 |
| 2 | 1:3 | 51 | 0:84:16 |
| 3 | 1:4 | 92 | 0:73:27 |
| 4 | 1:6 | 92 | 0:69:31 |

| Entry | Ionic Liquid | Isolated Yield (%) | Ratio (3:4a:4b) % |
|---|---|---|---|
| 1 a | [BMIM][BF4] | 88 | 100:0:0 |
| 2 | [BPy][Br]27 | 77 | 0:76:24 |
| 3 a | [BMIM][PF6] | 75 | 0:73:27 |
| 4 a | [BMIM][CF3CO2] | 48 | 100:0:0 |
| 5 a | [BMIM][CF3SO3] | 85 | 100:0:0 |

| Entry a | Metallic Sources | Conversion (%) c | Ratio 3:4a:4b |
|---|---|---|---|
| 1 | SnCl2·2H2O | 92 | 0:73:27 |
| 2 | Zn | 0 | 0:0:0 |
| 3 | Sn | 0 | 0:0:0 |
| 4 | SnCl2·2H2O/SnCl4 | 85 | 0:82:18 |
| 5 b | SnCl2·2H2O/AlCl3 | 86 | 0:83:17 |
| 6 | SnBr2 | 90 | 0:0:100 |
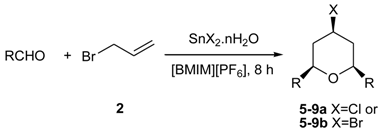
| Entry | Aldehyde | Tin Halide | Yield Isolated (%) | Ratio |
|---|---|---|---|---|
| 1 a |  | SnCl2.2H2O | 75 | 4a:4b (73:27) |
| 2 a | 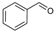 | SnBr2 | 73 | 4b |
| 3 a |  | SnCl2.2H2O | 71 | 5a:5b (63:37) |
| 4 a | 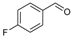 | SnBr2 | 70 | 5b |
| 5 a |  | SnCl2.2H2O | 71 | 6a:6b (62:38) |
| 6 a |  | SnBr2 | 72 | 6b |
| 7 a | 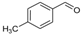 | SnCl2.2H2O | 60 | 7a:7b (65:35) |
| 8 a |  | SnBr2 | 62 | 7b |
| 9 b | 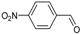 | SnCl2.2H2O | 40 | 8a:8b (65:35) |
| 10 b | 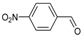 | SnBr2 | 45 | 8b |
| 11 a |  | SnCl2.2H2O | 61 | 9a:9b(60:40) |
| 12 a |  | SnBr2 | 60 | 9b |
| Entry | Solvent | Yield Isolated (%) |
|---|---|---|
| 1 | - | 0 |
| 2 | H2O/0.5 mL | 43 |
| 3 | H2O/10 µL | 63 |
| 4 | Toluene/0.5 mL | 0 |
| 5 | Ethanol/0.5 mL | 31 |
| 6 | Acetonitrile/0.5 mL | 34 |
| 7 | CH2Cl2/0.5 mL | 37 |

| Entry | Condition | Yield Isolated (%) |
|---|---|---|
| 1 | SnBr22H2O/KPF6 | 78 |
| 2 | SnBr2.2H2O | 77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, P.K.; de O. Ferreira, J.M.G.; Silva, F.P.L.; Vasconcellos, M.L.A.A.; Vale, J.A. The Role Ionic Liquid [BMIM][PF6] in One-Pot Synthesis of Tetrahydropyran Rings through Tandem Barbier–Prins Reaction. Molecules 2019, 24, 2084. https://doi.org/10.3390/molecules24112084
Batista PK, de O. Ferreira JMG, Silva FPL, Vasconcellos MLAA, Vale JA. The Role Ionic Liquid [BMIM][PF6] in One-Pot Synthesis of Tetrahydropyran Rings through Tandem Barbier–Prins Reaction. Molecules. 2019; 24(11):2084. https://doi.org/10.3390/molecules24112084
Chicago/Turabian StyleBatista, Poliane K., João Marcos G. de O. Ferreira, Fabio P. L. Silva, Mario L. A. A Vasconcellos, and Juliana A. Vale. 2019. "The Role Ionic Liquid [BMIM][PF6] in One-Pot Synthesis of Tetrahydropyran Rings through Tandem Barbier–Prins Reaction" Molecules 24, no. 11: 2084. https://doi.org/10.3390/molecules24112084







