Optimization of Ultrasonic-Assisted Extraction of Total Phenolics from Citrus aurantium L. Blossoms and Evaluation of Free Radical Scavenging, Anti-HMG-CoA Reductase Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experimental Analysis
2.1.1. Effect of Ethanol Concentration on the Extraction Yield of Total Phenolics
2.1.2. Effect of Extraction Temperature on Extraction Yield of Total Phenolics
2.1.3. Effect of Extraction Time on Extraction Yield of Total Phenolics
2.1.4. Effect of Liquid-to-Solid Ratio on Extraction Yield of Total Phenolics
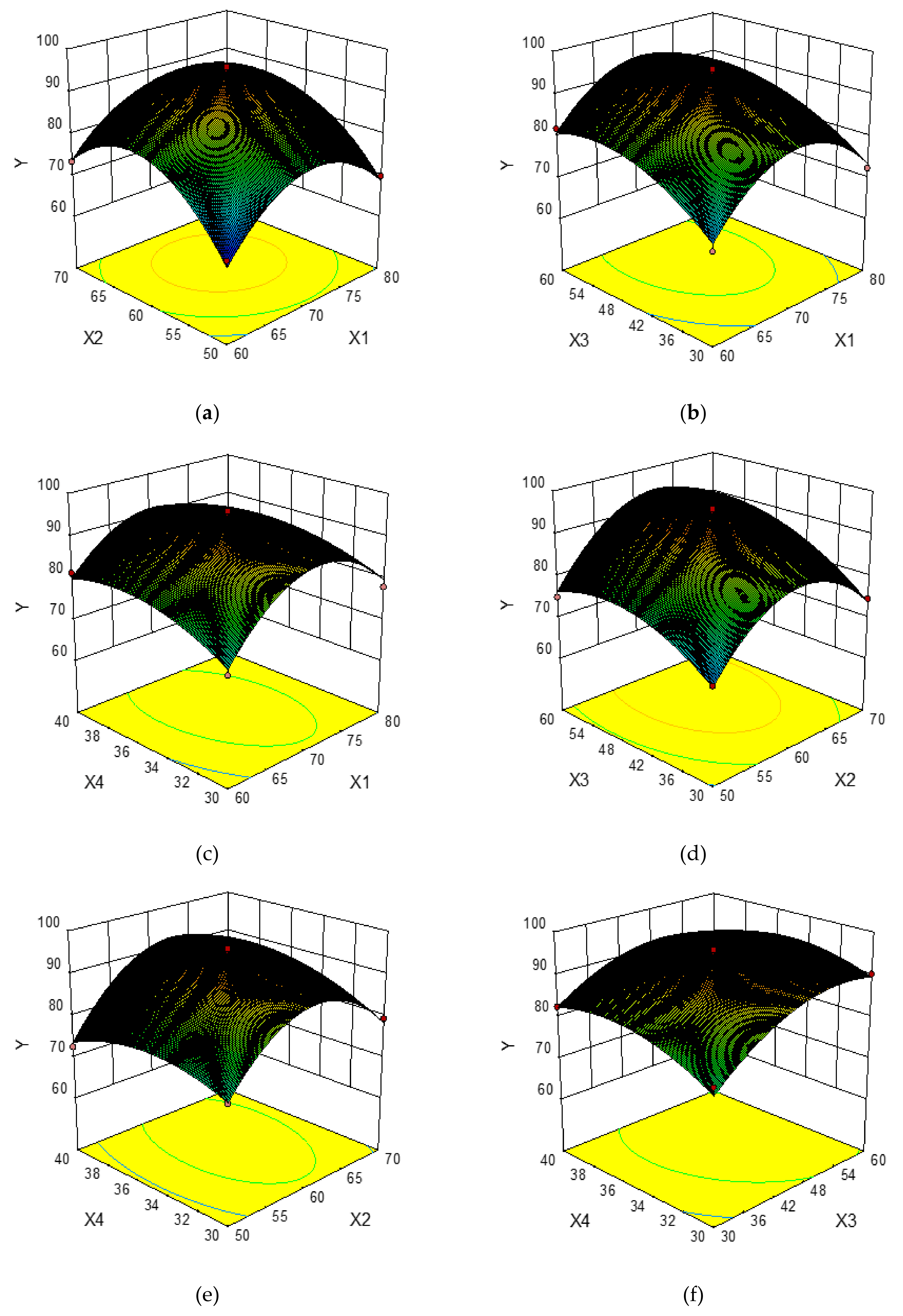
2.2. Optimization of Variables by Box–Behnken Design
2.2.1. Statistical Analysis and Model Fitting
2.2.2. Optimization of UAE Procedure
2.2.3. Verification of Predictive Model
2.3. Comparison of UAE and HRE
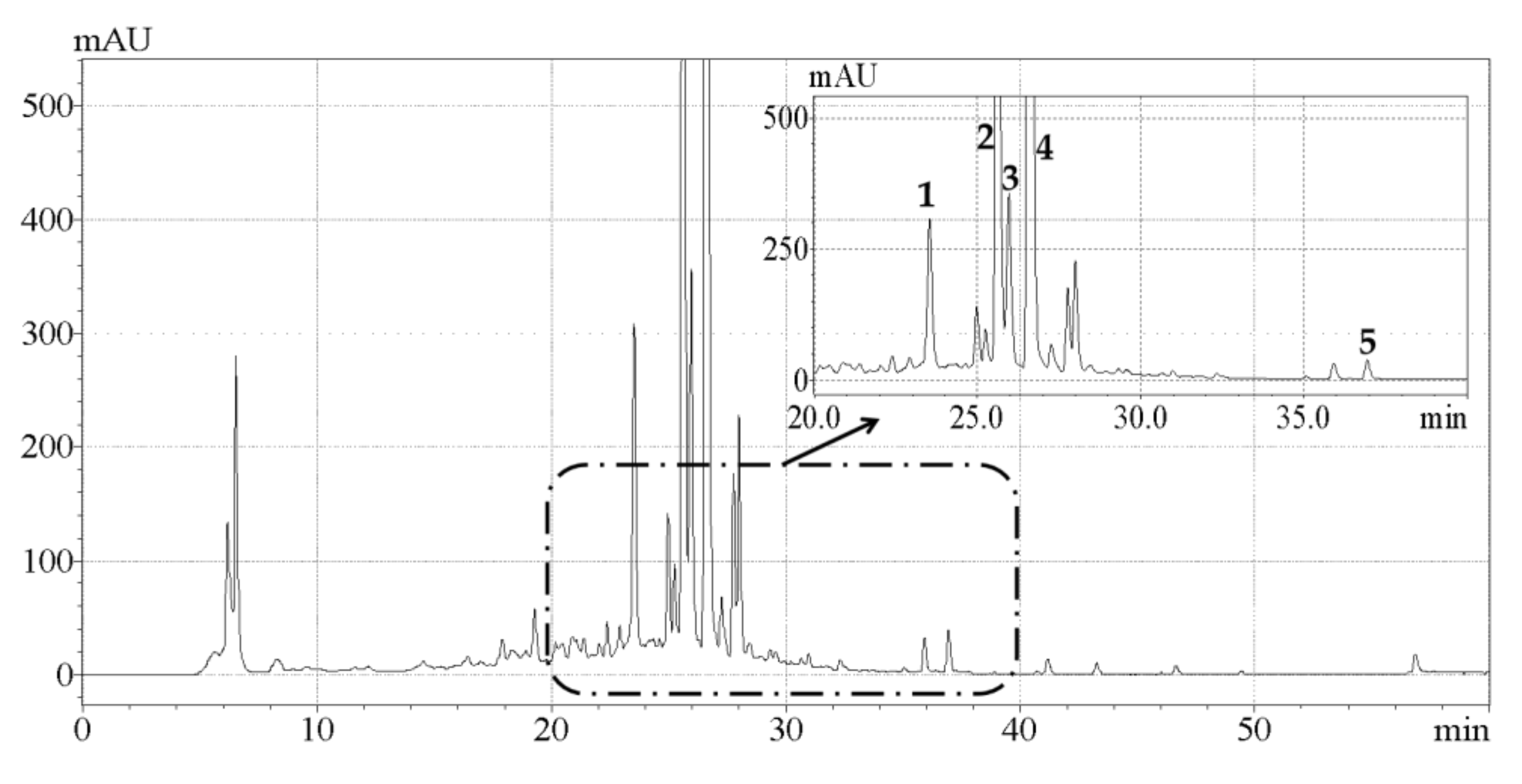
2.4. Analysis of Phenolic Compounds by HPLC
2.5. Evaluation of Free Radical Scavenging Activities
2.5.1. DPPH· Scavenging Activity
2.5.2. ABTS+· Scavenging Activity
2.5.3. ·OH Scavenging Activity
2.5.4. ·O2− Scavenging Activity
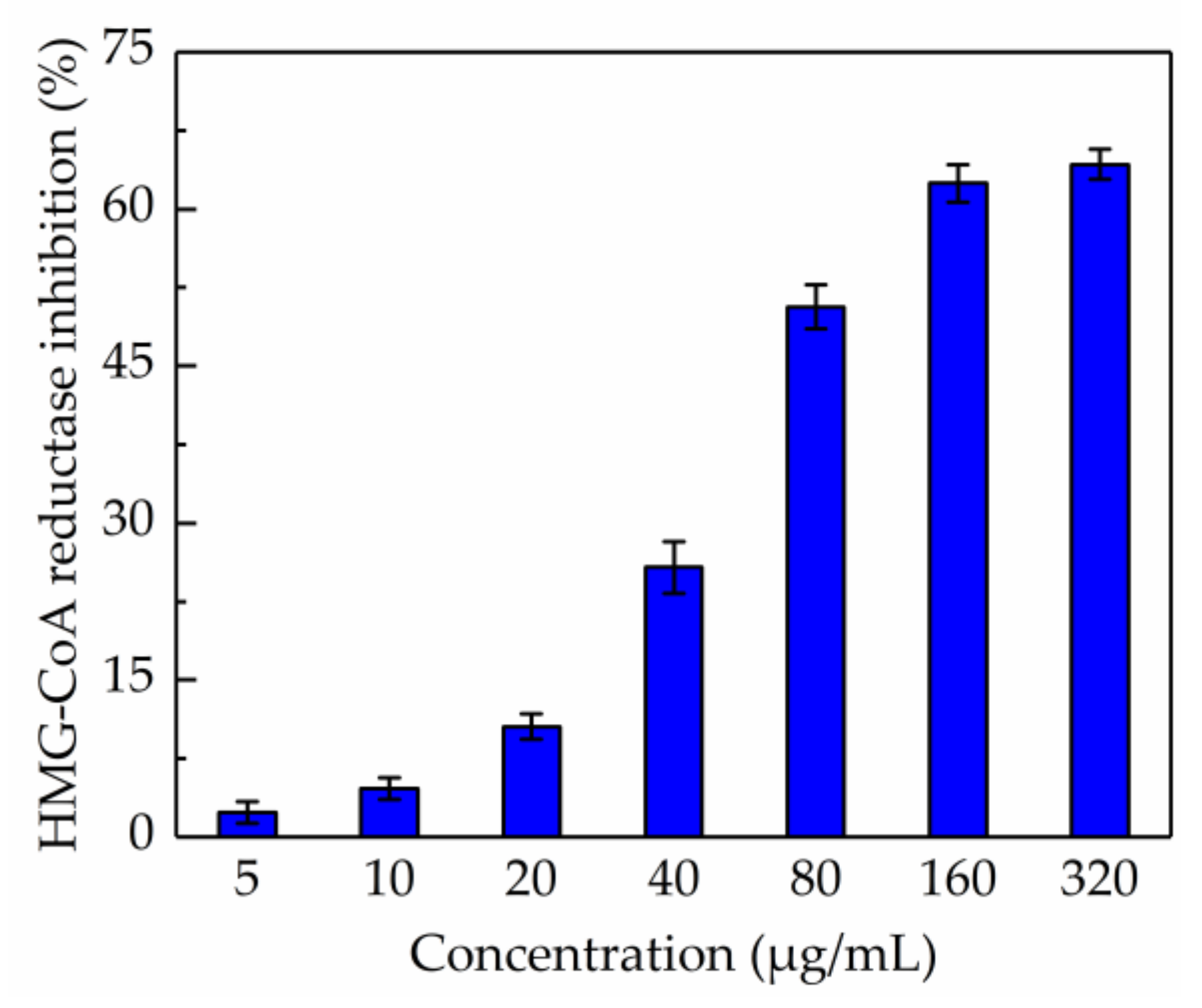
2.6. Evaluation of Anti-HMG-CoA Reductase Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. UAE of Total Phenolics
3.3.1. Single-Factor Experiments
3.3.2. Box–Behnken Design
3.4. HRE of Total Phenolics from CAB
3.5. Determination of Total Phenolic Content
3.6. HPLC Analysis
3.7. Free Radical Scavenging Activity Assay
3.7.1. DPPH· Scavenging Activity
3.7.2. ABTS+· Scavenging Activity Assay
3.7.3. ·OH Scavenging Activity Assay
3.7.4. ·O2− Scavenging Activity Assay
3.8. Anti-HMG-CoA Reductase Activity Assay
3.9. Statistical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Bioche. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food antioxidants and their anti-inflammatory properties: A potential role in cardiovascular diseases and cancer prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Ogura, M.; Hori, M.; Harada-Shiba, M. Association Between cholesterol efflux capacity and atherosclerotic cardiovascular disease in patients with familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 181–188. [Google Scholar] [CrossRef]
- Brown, M.; Goldstein, J. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 1980, 21, 505–517. [Google Scholar] [PubMed]
- Carbonell, T.; Freire, E. Binding thermodynamics of statins to HMG-CoA reductase. Biochemistry 2005, 44, 11741–11748. [Google Scholar] [CrossRef] [PubMed]
- Kiortsis, D.N.; Filippatos, T.D.; Mikhailidis, D.P.; Elisaf, M.S.; Liberopoulos, E.N. Statin-associated adverse effects beyond muscle and liver toxicity. Atherosclerosis 2007, 195, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An overview on citrus aurantiuml.: Its functions as food ingredient and therapeutic agent. Oxidative Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Jiang, J.G.; Huang, C.L.; Huang, C.L.; Zhu, W.; Zheng, C.Y. Polyphenols from blossoms of Citrus aurantium L. var. amara Engl. show significant anti-complement and anti-inflammatory effects. J. Agric. Food Chem. 2017, 65, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H. Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules 2012, 17, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Wang, T.X.; Zhang, X.M.; Jiang, J.G. Various antioxidant effects were attributed to different components in the dried blossoms of Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 6087–6092. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, Y.L.; Jiang, J.G.; Lin, Q.S.; Chen, J.; Zhu, L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [PubMed]
- Shen, C.Y.; Wang, T.X.; Jiang, J.G. Extraction optimization and adsorption isotherm kinetics of polyphenols from blossoms of Citrus aurantium L. var. amara Engl. Sep. Sci. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Boggia, R.; Leardi, R.; Borriello, M.; Zunin, P. Optimization of the ultrasonic-assisted extraction of phenolic compounds from Oryza sativa L. ‘Violet Nori’ and determination of the antioxidant properties of its caryopses and leaves. Molecules 2018, 23, 844. [Google Scholar] [CrossRef] [PubMed]
- Gullian Klanian, M.; Terrats Preciat, M. Optimization of the ultrasound-assisted extraction of phenolic compounds from Brosimum alicastrum leaves and the evaluation of their radical-scavenging activity. Molecules 2017, 22, 1286. [Google Scholar] [CrossRef]
- Yang, L.; Yin, P.; Fan, H.; Xue, Q.; Li, K.; Li, X.; Sun, L.; Liu, Y. Response surface methodology optimization of ultrasonic-assisted extraction of Acer truncatum leaves for maximal phenolic yield and antioxidant activity. Molecules 2017, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Chimuka, L.; Michel, M.; Cukrowska, E.; Buszewski, B. Influence of temperature on mass transfer in an incomplete trapping supported liquid membrane extraction of triazole fungicides. J. Sep. Sci. 2009, 32, 1043–1050. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Feng, S.; Luo, Z.; Tao, B.; Chen, C. Ultrasonic-assisted extraction and purification of phenolic compounds from sugarcane (Saccharum officinarum L.) rinds. LWT-Food Sci. Technol. 2015, 60, 970–976. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Liu, Y.; Wu, Y.; Wu, Z. Ultrasound extraction of polysaccharides from guava leaves and their antioxidant and antiglycation activity. Process Biochem. 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Chemat, F.; Grondin, I.; Costes, P.; Moutoussamy, M.; Shum Cheong Sing, A.; Smadja, J. High power ultrasound effects on lipid oxidation of refined sunflower oil. Ultrason. Sonochem. 2004, 11, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre-and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Iqbal, D.; Khan, M.S.; Khan, A.; Khan, M.S.; Ahmad, S.; Srivastava, A.K.; Bagga, P. In vitro screening for β-hydroxy-β-methylglutaryl-CoA reductase inhibitory and antioxidant activity of sequentially extracted fractions of Ficus palmata Forsk. Biomed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.Y.; Zhou, G.M.; Peng, G.L.; Li, J.P.; Chen, J.H. Application of ionic liquid-based ultrasound-assisted extraction of five phenolic compounds from Fig (Ficus carica L.) for HPLC-UV. Food Anal. Meth. 2015, 1673–1681. [Google Scholar] [CrossRef]
- Goltz, C.; Ávila, S.; Barbieri, J.B.; Igarashi-Mafra, L.; Mafra, M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind. Crop. Prod. 2018, 115, 227–234. [Google Scholar] [CrossRef]
- Seifzadeh, N.; Ali Sahari, M.; Barzegar, M.; Ahmadi Gavlighi, H.; Calani, L.; Del Rio, D.; Galaverna, G. Evaluation of polyphenolic compounds in membrane concentrated pistachio hull extract. Food Chem. 2019, 277, 398–406. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef]
- Awe, F.B.; Fagbemi, T.N.; Ifesan, B.O.T.; Badejo, A.A. Antioxidant properties of cold and hot water extracts of cocoa, Hibiscus flower extract, and ginger beverage blends. Food Res. Int. 2013, 52, 490–495. [Google Scholar] [CrossRef]
- Su, X.Y.; Wang, Z.Y.; Liu, J.R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.A.M.; Mendonça, S.; de Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major peptides from amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Monakhova, Y.B.; Kuballa, T.; Löbell-Behrends, S.; Maixner, S.; Kohl-Himmelseher, M.; Waldner, A.; Steffen, C. NMR evaluation of total statin content and HMG-CoA reductase inhibition in red yeast rice (Monascus spp.) food supplements. Chin. Med. 2012, 7, 8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of CAB and total phenolic extract are available from the authors. |


| Run | Independent Variables | Y (mg GAE/g DM) | ||||
|---|---|---|---|---|---|---|
| X1 (%) | X2 (°C) | X3 (min) | X4 (mL/g) | Experimental | Predicted | |
| 1 | 60 | 60 | 45 | 30 | 72.19 | 73.63 |
| 2 | 70 | 60 | 45 | 35 | 95.81 | 94.49 |
| 3 | 70 | 60 | 45 | 35 | 95.37 | 94.49 |
| 4 | 70 | 70 | 45 | 30 | 79.37 | 78.38 |
| 5 | 70 | 50 | 60 | 35 | 74.97 | 76.29 |
| 6 | 80 | 60 | 30 | 35 | 72.36 | 73.48 |
| 7 | 70 | 60 | 30 | 30 | 77.98 | 76.20 |
| 8 | 60 | 60 | 30 | 35 | 68.28 | 70.01 |
| 9 | 80 | 60 | 45 | 30 | 77.99 | 79.74 |
| 10 | 70 | 60 | 30 | 40 | 82.47 | 82.40 |
| 11 | 70 | 50 | 45 | 40 | 72.52 | 73.68 |
| 12 | 60 | 50 | 45 | 35 | 65.69 | 64.30 |
| 13 | 70 | 60 | 45 | 35 | 93.64 | 94.49 |
| 14 | 70 | 60 | 45 | 35 | 93.47 | 94.49 |
| 15 | 70 | 70 | 45 | 40 | 85.27 | 85.21 |
| 16 | 70 | 60 | 60 | 30 | 90.21 | 89.55 |
| 17 | 60 | 60 | 60 | 35 | 81.90 | 80.96 |
| 18 | 80 | 70 | 45 | 35 | 74.01 | 74.67 |
| 19 | 70 | 60 | 45 | 35 | 94.17 | 94.49 |
| 20 | 80 | 50 | 45 | 35 | 69.86 | 68.77 |
| 21 | 80 | 60 | 60 | 35 | 84.4 | 82.84 |
| 22 | 60 | 60 | 45 | 40 | 81.27 | 80.07 |
| 23 | 70 | 60 | 60 | 40 | 88.32 | 89.37 |
| 24 | 70 | 70 | 60 | 35 | 86.22 | 87.01 |
| 25 | 70 | 50 | 30 | 35 | 69.39 | 69.15 |
| 26 | 80 | 60 | 45 | 40 | 80.19 | 79.31 |
| 27 | 70 | 50 | 45 | 30 | 74.26 | 74.50 |
| 28 | 70 | 70 | 30 | 35 | 74.61 | 73.84 |
| 29 | 60 | 70 | 45 | 35 | 73.43 | 73.79 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2196.5259 | 14 | 156.8947 | 66.3436 | <0.0001 *** |
| X1 | 21.4669 | 1 | 21.4669 | 9.0774 | 0.0093 ** |
| X2 | 178.0240 | 1 | 178.0240 | 75.2782 | <0.0001 *** |
| X3 | 309.3721 | 1 | 309.3721 | 130.8193 | <0.0001 *** |
| X4 | 27.1201 | 1 | 27.1201 | 11.4679 | 0.0044 ** |
| X1X2 | 3.2220 | 1 | 3.2220 | 1.3624 | 0.2626 |
| X1X3 | 0.6241 | 1 | 0.6241 | 0.2639 | 0.6154 |
| X1X4 | 11.8336 | 1 | 11.8336 | 5.0039 | 0.0421 * |
| X2X3 | 9.0902 | 1 | 9.0902 | 3.8438 | 0.0701 |
| X2X4 | 14.5924 | 1 | 14.5924 | 6.1705 | 0.0263 * |
| X3X4 | 10.1761 | 1 | 10.1761 | 4.3030 | 0.0570 |
| X12 | 923.4078 | 1 | 923.4078 | 390.4671 | <0.0001 *** |
| X22 | 961.9172 | 1 | 961.9172 | 406.7509 | <0.0001 *** |
| X32 | 213.6335 | 1 | 213.6335 | 90.3359 | <0.0001 *** |
| X42 | 124.0230 | 1 | 124.0230 | 52.4437 | <0.0001 *** |
| Residual | 33.1083 | 14 | 2.3649 | ||
| Lack of Fit | 28.7262 | 10 | 2.8726 | 2.6222 | 0.1829 |
| Pure Error | 4.3821 | 4 | 1.0955 | ||
| Cor Total | 2229.6342 | 28 |
| Extraction Method | Content of Individual Phenolic Compounds (mg/g) | ||||
|---|---|---|---|---|---|
| Eriocitrin | Naringin | Hesperidin | Neohesperidin | Hesperetin | |
| UAE | 1.62 ± 0.03 | 6.17 ± 0.04 | 10.22 ± 0.03 | 15.45 ± 0.05 | 0.68 ± 0.03 |
| HRE | 1.17 ± 0.02 | 5.03 ± 0.03 | 8.25 ± 0.02 | 11.77 ± 0.03 | 0.56 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, K.; Hu, W.; Hou, M.; Cao, D.; Wang, Y.; Guan, Q.; Zhang, X.; Wang, A.; Yu, J.; Guo, B. Optimization of Ultrasonic-Assisted Extraction of Total Phenolics from Citrus aurantium L. Blossoms and Evaluation of Free Radical Scavenging, Anti-HMG-CoA Reductase Activities. Molecules 2019, 24, 2368. https://doi.org/10.3390/molecules24132368
Hao K, Hu W, Hou M, Cao D, Wang Y, Guan Q, Zhang X, Wang A, Yu J, Guo B. Optimization of Ultrasonic-Assisted Extraction of Total Phenolics from Citrus aurantium L. Blossoms and Evaluation of Free Radical Scavenging, Anti-HMG-CoA Reductase Activities. Molecules. 2019; 24(13):2368. https://doi.org/10.3390/molecules24132368
Chicago/Turabian StyleHao, Kexin, Wenzhong Hu, Mengyang Hou, Duo Cao, Yu Wang, Qingxin Guan, Xiufu Zhang, Aosheng Wang, Jiaoxue Yu, and Binmei Guo. 2019. "Optimization of Ultrasonic-Assisted Extraction of Total Phenolics from Citrus aurantium L. Blossoms and Evaluation of Free Radical Scavenging, Anti-HMG-CoA Reductase Activities" Molecules 24, no. 13: 2368. https://doi.org/10.3390/molecules24132368
APA StyleHao, K., Hu, W., Hou, M., Cao, D., Wang, Y., Guan, Q., Zhang, X., Wang, A., Yu, J., & Guo, B. (2019). Optimization of Ultrasonic-Assisted Extraction of Total Phenolics from Citrus aurantium L. Blossoms and Evaluation of Free Radical Scavenging, Anti-HMG-CoA Reductase Activities. Molecules, 24(13), 2368. https://doi.org/10.3390/molecules24132368





