Euphosantianane E–G: Three New Premyrsinane Type Diterpenoids from Euphorbia sanctae-catharinae with Contribution to Chemotaxonomy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of the Isolated Compounds
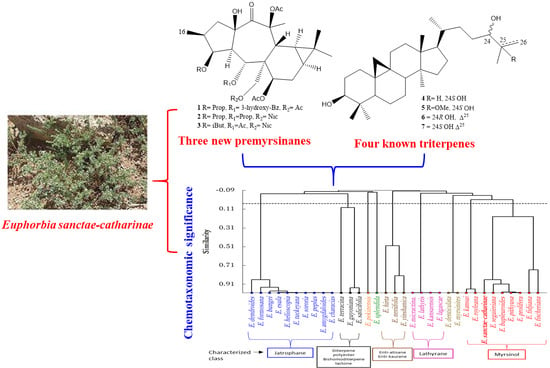
2.2. Study of Chemosystematic Significance
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Spectroscopic Data of Euphosantianane E–G (1–3)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eissa, T.; Palomino, O.; Carretero, M.; Gómez-Serranillos, M. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014, 151, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Flora of Egypt Checklist; Al Hadara Publishing: Cairo, Egypt, 2009. [Google Scholar]
- Batanouny, K.H.; Aboutabl, E.; Shabana, M.F.S. Wild Medicinal Plants in Egypt. An Inventory to Support Conservation and Sustainable Use; The Palm Press: Cairo, Egypt, 1999. [Google Scholar]
- Boulos, L.; Gibali, M. List of rare, vulnerable, endangered and endemic species of vascular plants in Sinai Peninsula. In Proceedings of the First Egypt-Hungary Conference on Environment, Cairo, Egypt, 5–7 April 1993; pp. 275–282. [Google Scholar]
- Barakat, N.; Abd El-Gawad, A.; Laudadio, V.; Kabiel, H.; Tufarelli, V.; Cazzato, E. A contribution to the ecology and floristic markers of plant associations in different habitats of Sinai Peninsula, Egypt. Rendiconti Lincei Scienze Fisiche e Naturali 2014, 25, 479–490. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodiv. 2019, 16, e1900051. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.; Hamed, A.; Ibrahim, M.; Talat, Z.; Reda, E.; Abdel-Azim, N.; Hammouda, F.; Nakamura, S.; Matsuda, H.; Haggag, E. Euphosantianane A–D: Antiproliferative premyrsinane diterpenoids from the endemic Egyptian plant Euphorbia sanctae-catharinae. Molecules 2018, 23, 2221. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Yu, L.; Tang, Y.; Zhang, L.; Ding, A.; Luo, D.; Duan, J.-a.; Shen, X. Bioassay-guided separation of the proinflammatory constituents from the roots of Euphorbia kansui. J. Nat. Med. 2010, 98, 64–103. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Khan, A.-U.; Chaudhary, B.A.; Janbaz, K.H.; Uzair, M.; Akhtar, M.; Gilani, A.-H. Antifungal and antispasmodic activities of the extracts of Euphorbia granulata. J. Med. Plants Res. 2012, 6, 19–23. [Google Scholar]
- Esposito, M.L.; Nothias, L.-F.L.; Retailleau, P.; Costa, J.; Roussi, F.; Neyts, J.; Leyssen, P.; Touboul, D.; Litaudon, M.; Paolini, J. Isolation of premyrsinane, myrsinane, and tigliane diterpenoids from Euphorbia pithyusa using a Chikungunya virus cell-based assay and analogue annotation by molecular networking. J. Nat. Prod. 2017, 80, 2051–2059. [Google Scholar] [CrossRef]
- Pracheta, S.V.; Paliwal, R.; Sharma, S. Preliminary phytochemical screening and in vitro antioxidant potential of hydro-ethanolic extract of Euphorbia neriifolia Linn. Int. J. PharmTech Res. 2011, 3, 124–132. [Google Scholar]
- Ibraheim, Z.Z.; Ahmed, A.S.; Abdel-Mageed, W.M. Chemical and biological studies of Euphorbia aphylla. J. Nat. Rem. 2013, 13, 35–45. [Google Scholar]
- Wu, L.; Zhou, P.J.; Wang, X.F. The application of antibacterial components of Euphorbia Humifusa Willd on silk fabrics. Adv. Mater. Res. 2012, 441, 315–319. [Google Scholar] [CrossRef]
- Lirio, L.; Hermano, M.; Fontanilla, M. Note antibacterial activity of medicinal plants from the Philippines. Pharm. Biol. 1998, 36, 357–359. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Xie, C.; Li, Y.; Gao, J.; Zhang, T.; Hou, W.; Fang, L.; Gui, L. Bioactive myrsinol diterpenoids from the roots of Euphorbia prolifera. J. Nat. Prod. 2011, 74, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Belloro, E.; Tron, G.C.; Jakupovic, J.; Ballero, M. Diterpenoids from Euphorbia pithyusa subsp. cupanii. J. Nat. Prod. 1999, 62, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, E.; Mayumi, M.; Atsuko, O.; Miwako, H.; Hiroyuki, Y. A new method for the determination of absolute configuration of isolated hydroxyl groups: CD spectra of acetates and methoxyglyoxalates. Proc. Symp. Chem. Nat. Prod. 1991, 33, 448–455. [Google Scholar]
- Kikuchi, T.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Watanabe, K.; Nishino, H. Cancer chemopreventive effects of cycloartane-type and related triterpenoids in in vitro and in vivo models. J. Nat. Prod. 2007, 70, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Scobedo-Martínez, C.; Concepción Lozada, M.; Hernández-Ortega, S.; Villarreal, M.L.; Gnecco, D.; Enríquez, R.G.; Reynolds, W. 1H and 13C-NMR characterization of new cycloartane triterpenes from Mangifera indica. Magn. Reson. Chem. 2012, 50, 52–57. [Google Scholar] [CrossRef]
- Chunhui, M.; Tianfang, H.; Huayi, Q.; Bogang, L.; Guolin, Z. Chemical study of Streptocaulon griffithii. Chin. J. Appl. Environ. Biol. 2005, 11, 265–270. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2000; Volume 2. [Google Scholar]
- Corea, G.; Fattorusso, C.; Fattorusso, E.; Lanzotti, V. Amygdaloidins A–L, twelve new 13 α-OH jatrophane diterpenes from Euphorbia amygdaloides L. Tetrahedron 2005, 61, 4485–4494. [Google Scholar] [CrossRef]
- Yu, C.-C.; Hsieh, C.-R.; Hsiao, G.; Chen, P.-Y.; Chang, M.-L.; Yin, H.-W.; Lee, T.-H.; Lee, C.-K. Regulated expressions of mmp-2,-9 by diterpenoids from Euphorbia formosana hayata. Molecules 2012, 17, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Helmboldt, H.; Hiersemann, M. Synthetic studies toward jatrophane diterpenes from Euphorbia characias. Enantioselective synthesis of (−)-15-O-Acetyl-3-O-propionyl-17-norcharaciol. J. Org. Chem. 2009, 74, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Corea, G.; Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Appendino, G.; Ballero, M.; Simon, P.-N.; Dumontet, C.; Di Pietro, A. Modified jatrophane diterpenes as modulators of multidrug resistance from Euphorbia dendroides L. Bioorg. Med. Chem. 2003, 11, 5221–5227. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.; Banković, J.; Aljančić, I.S.; Todorović, N.M.; Jadranin, M.; Vajs, V.E.; Tešević, V.V.; Vučković, I.; Momčilović, M.; Marković, I.D. New anti-cancer characteristics of jatrophane diterpenes from Euphorbia dendroides. Food Chem. Toxicol. 2011, 49, 3165–3173. [Google Scholar] [CrossRef]
- Jadranin, M.; Pešić, M.; Aljančić, I.S.; Milosavljević, S.M.; Todorović, N.M.; Podolski-Renić, A.; Banković, J.; Tanić, N.; Marković, I.; Vajs, V.E. Jatrophane diterpenoids from the latex of Euphorbia dendroides and their anti-P-glycoprotein activity in human multi-drug resistant cancer cell lines. Phytochemistry 2013, 86, 208–217. [Google Scholar] [CrossRef]
- Manners, G.D.; Wong, R.Y. The absolute stereochemical characterization of two new jatrophane diterpenes from Euphorbia esula. J. Chem. Soc. Perkin Trans. 1985, 1, 2075–2081. [Google Scholar] [CrossRef]
- Corea, G.; Di Pietro, A.; Dumontet, C.; Fattorusso, E.; Lanzotti, V. Jatrophane diterpenes from Euphorbia spp. as modulators of multidrug resistance in cancer therapy. Phytochem. Rev. 2009, 8, 431–447. [Google Scholar] [CrossRef]
- Kosemura, S.; Shizuri, Y.; Yamamura, S. Isolation and structures of euphohelins, new toxic diterpenes from Euphorbia helioscopia L. Bull. Chem. Soc. Jpn. 1985, 58, 3112–3117. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Yang, B.; Jin, D.-Q.; Yang, S.; Wang, M.; Xu, J.; Ohizumi, Y.; Guo, Y. Diterpenes inhibiting NO production from Euphorbia helioscopia. Fitoterapia 2014, 95, 133–138. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.-W. Three new jatrophone-type diterpenoids from Euphorbia helioscopia. Planta Med. 2005, 71, 283–286. [Google Scholar] [CrossRef]
- Huang, Y.; Aisa, H.A. Three new diterpenoids from Euphorbia sororia L. Helv. Chim. Acta 2010, 93, 1156–1161. [Google Scholar] [CrossRef]
- Duarte, N.; Lage, H.; Ferreira, M.-J.U. Three new jatrophane polyesters and antiproliferative constituents from Euphorbia tuckeyana. Planta Med. 2008, 74, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.; Varga, A.; Cherepnev, G.; Radics, R.; Molnár, J.; Ferreira, M.-J.U. Apoptosis induction and modulation of P-glycoprotein mediated multidrug resistance by new macrocyclic lathyrane-type diterpenoids. Bioorg. Med. Chem. 2007, 15, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Dong, W.; Li, Z.; Deng, M.; Lu, R. Lathyrane diterpenes from Euphorbia lathyris as modulators of multidrug resistance and their crystal structures. Bioorg. Med. Chem. 2009, 17, 4786–4792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Liang, Y.-J.; Chen, H.-B.; Zheng, L.-S.; Mi, Y.-J.; Wang, F.; Zhao, X.-Q.; Wang, X.-K.; Zhang, H.; Fu, L.-W. Structure identification of Euphorbia factor L3 and its induction of apoptosis through the mitochondrial pathway. Molecules 2011, 16, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ye, D.; Wang, Y.; Zhao, Y.; Pu, J.; Du, X.; Luo, L.; Zhao, Y. Ent-Kaurane diterpenoids from Euphorbia hirta. Rec. Nat. Prod. 2011, 5, 247–251. [Google Scholar]
- Liu, J.-H.; Latif, A.; Ali, M.; Zhang, G.-P.; Xiang, W.-J.; Ma, L.; Arfan, M.; Hu, L.-H. Diterpenoids from Euphorbia neriifolia. Phytochemistry 2012, 75, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Wang, H.; Luo, X.D.; Du, Z.Z.; Shen, J.W.; Wu, H.F.; Zhang, X.F. Bisyinshanic acids A and B, two novel diterpene dimers from the roots of Euphorbia yinshanica. Helv. Chim. Acta 2012, 95, 1672–1679. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz-Cervera, J.F.; Yuste, A.; Jakupovic, J.; Lex, J. Terracinolides A and B, two bishomoditerpene lactones with a novel carbon framework from Euphorbia terracina. J. Org. Chem. 1996, 61, 1707–1709. [Google Scholar] [CrossRef]
- El-Bassuony, A.A. Antibacterial activity of new polyester diterpenes from Euphorbia guyoniana. Asian J. Chem. 2007, 19, 4553–4562. [Google Scholar]
- Hegazy, M.-E.F.; Mohamed, A.E.-H.H.; Aoki, N.; Ikeuchi, T.; Ohta, E.; Ohta, S. Bioactive jatrophane diterpenes from Euphorbia guyoniana. Phytochemistry 2010, 71, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, J.; Evanics, F.; Dombi, G.; Molnár, J.; Szabó, P. Euphosalicin, a new diterpene polyester with multidrug resistance reversing activity from Euphorbia salicifolia. Tetrahedron 2001, 57, 211–215. [Google Scholar] [CrossRef]
- Xu, J.; Jin, D.; Guo, P.; Xie, C.; Fang, L.; Guo, Y. Three new myrsinol diterpenes from Euphorbia prolifera and their neuroprotective activities. Molecules 2012, 17, 9520–9528. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, D.-q.; Guo, Y.; Xie, C.; Ma, Y.; Yamakuni, T.; Ohizumi, Y. New myrsinol diterpenes from Euphorbia prolifera and their inhibitory activities on LPS-induced NO production. Bioorg. Med. Chem. Lett. 2012, 22, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, D.-q.; Song, H.; Guo, Y.; He, Y. Lathyrane diterpenes from Euphorbia prolifera and their inhibitory activities on LPS-induced NO production. Fitoterapia 2012, 83, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Jeske, F.; Jakupovic, J.; Berendsohn, W. Diterpenes from Euphorbia seguieriana. Phytochemistry 1995, 40, 1743–1750. [Google Scholar] [CrossRef]
- Dagang, W.; Sorg, B.; Hecker, E. Oligo-and macrocyclic diterpenes in thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China). 6. Tigliane type diterpene esters from latex of Euphorbia prolifera. Phytother. Res. 1994, 8, 95–99. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, F.P. New cytotoxic myrsinane-type diterpenes from Euphorbia prolifera. Helv. Chim. Acta 2010, 93, 746–752. [Google Scholar] [CrossRef]
- Öksüz, S.; Gürek, F.h.; Gil, R.R.; Pengsuparp, T.; Pezzuto, J.M.; Cordell, G.A. Four diterpene esters from Euphorbia myrsinites. Phytochemistry 1995, 38, 1457–1462. [Google Scholar] [CrossRef]
- Shamsabadipour, S.; Ghanadian, M.; Saeedi, H.; Rahimnejad, M.R.; Mohammadi-Kamalabadi, M.; Ayatollahi, S.M.; Salimzadeh, L. Triterpenes and steroids from Euphorbia denticulata Lam. with anti-Herpes symplex virus activity. Iran. J. Pharm. Res. 2013, 12, 759–767. [Google Scholar]
- Ghannadian, M.; Akhavan, A.; Abdalla, O.; Ayatollahi, A.; Mohammadi-Kamalabadi, M.; Ghazanfari, H. Triterpenes from Euphorbia spinidens with immunomodulatory activity. Res. Pharm. Sci. 2013, 8, 205–210. [Google Scholar] [PubMed]
- Li, X.-L.; Li, Y.; Wang, S.-F.; Zhao, Y.-L.; Liu, K.-C.; Wang, X.-M.; Yang, Y.-P. Ingol and ingenol diterpenes from the aerial parts of Euphorbia royleana and their antiangiogenic activities. J. Nat. Prod. 2009, 72, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Khiev, P.; Kim, J.W.; Sung, S.J.; Song, H.-H.; Choung, D.-H.; Chin, Y.-W.; Lee, H.-K.; Oh, S.-R. Ingenane-type diterpenes with a modulatory effect on IFN-γ production from the roots of Euphorbia kansui. Arch. Pharm. Res. 2012, 35, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Lee, S.W.; Park, M.H.; Kim, M.S.; Hudson, B.I.; Park, S.-J.; Lee, W.S.; Rho, M.-C. Kansuinine A and Kansuinine B from Euphorbia kansui L. inhibit IL-6-induced Stat3 activation. Planta Med. 2010, 76, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Azmi, L.; Mohapatra, P.; Rao, C.V. Flavonoids from whole plant of Euphorbia hirta and their evaluation against experimentally induced gastroesophageal reflux disease in rats. Pharmacogn. Magaz. 2017, 13, 127–134. [Google Scholar]
- Hassan, G.F.; Omer, M.A.; Babadoust, S.; Najat, D.D. Flavonoids from Euphorbia condylocarpa roots. Int. J. Chem. Biochem. Sci. 2014, 6, 56–60. [Google Scholar]
- Kawashty, S.; Abdalla, M.; El-Hadidi, M.; Saleh, N. The chemosystematics of Egyptian Euphorbia species. Biochem. Syst. Ecol. 1990, 18, 487–490. [Google Scholar] [CrossRef]
- Ulubelen, A.; Öksüz, S.; Halfon, B.; Aynehchi, Y.; Mabry, T.J. Flavonoids from Euphorbia larica, E. virgata, E. chamaesyce and E. magalanta. J. Nat. Prod. 1983, 46, 598. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–7 are available or not from the authors. |




| No. | Euphosantianane E (1) | Euphosantianane F (2) | Euphosantianane G (3) | |||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |||
| 1α | 3.15 dd (8.05, 13.86) | 42.8, t | 2.64 dd (10.85, 14.64) | 41.1, t | 3.16 dd (8.10, 13.62) | 42.8, t | ||
| 1β | 1.64 t (13.26) | 1.60 m | 1.61 m | |||||
| 2 | 2.26 qd (1.56, 7.56) | 35.1, d | 2.31 m | 33.8, d | 2.25 m | 34.0, d | ||
| 3 | 5.35 br t (3.48) | 78.2, d | 5.46 br dd (3.06, 6.12) | 78.7, d | 5.20 s | 78.6, d | ||
| 4 | 2.37 dd (3.78, 11.5) | 50.1, d | 3.01 dd (3.30, 10.50) | 50.4, d | 2.37 brd (11.6) | 50.4, d | ||
| 5 | 6.42 d (11.46) | 70.5, d | 5.79 d (8.80) | 68.5, d | 6.23 d (11.52) | 69.0, d | ||
| 6 | ----- | 47.8, s | ----- | 48.2, s | ----- | 47.7, s | ||
| 7 | 4.77 d (6.72) | 70.6, d | 4.90 d (6.54) | 68.8, d | 4.68 d (6.54) | 70.7, d | ||
| 8α | 3.50 br d (6.54) | 22.1, t | 2.34 d (7.44) | 23.2, t | 2.32 d (7.14) | 22.4, d | ||
| 8β | 1.84 t (16.92) | 1.72 t (13.92) | 1.88 br d (17.40) | |||||
| 9 | 0.73 m | 18.9, d | 0.75 m | 19.4, d | 0.79 m | 19.0, d | ||
| 10 | ----- | 18.4, s | ----- | 19.1, s | ----- | 18.4, s | ||
| 11 | 0.73 m | 23.8, d | 0.75 m | 23.3, d | 0.79 m | 23.9, d | ||
| 12 | 3.48 s | 37.4, d | 3.47 br d (6.11) | 36.0, d | 3.45 br d (3.42) | 35.0, d | ||
| 13 | ----- | 85.7, s | ----- | 79.8, s | ----- | 85.5, s | ||
| 14 | ----- | 204.1, s | ----- | 210.2, s | ----- | 204.2, s | ||
| 15 | ----- | 84.2, s | ----- | 83.6, s | ----- | 84.1, s | ||
| 16 | 1.04 s | 13.9, q | 0.92 d (5.01) | 15.2, q | 0.77 d (5.04) | 14.2, q | ||
| 17α | 4.35 d (11.70) | 62.8, t | 4.55 d (12.18) | 62.6, t | 4.49 d (11.82) | 64.4, t | ||
| 17β | 4.67 d (11.70) | 5.28 d (12.30) | 5.86 d (11.94) | |||||
| 18 | 0.94 s | 29.5, q | 1.07 s | 28.8, q | 1.05 s | 29.6, q | ||
| 19 | 1.04 s | 14.9, q | 0.94 s | 15.3, q | 0.92 s | 14.9, q | ||
| 20 | 1.57 s | 25.0, q | 1.57 s | 23.0, q | 1.72 s | 24.6, q | ||
| 3-O-Prop | 3-O-Prop | 3-O-iBut | ||||||
| CO | ----- | 173.5, s | CO | ----- | 172.9 | CO | ----- | 175.1, s |
| 2` | 2.26 qd (7.62) | 27.5, t | 2` | 2.32 q (7.14) | 27.6, t | 2` | 2.15 m | 27.6, d |
| 3` | 0.98 t (7.5) | 8.8, q | 3` | 1.15 t (6.66) | 8.8, q | 3` | 1.05 d (7.02) | 8.9, q |
| 5-O-3-hydroxy-Bz | 5-O-Prop | 4` | 1.05 d (7.02) | 8.9, q | ||||
| CO | ----- | 168.9, s | CO | ----- | 170.1, s | 5-O-Ac | ||
| 2`` | ----- | 129.6, s | 2`` | 2.36 q (7.14) | 27.6, t | CO | ----- | 170.1, s |
| 3`` | 7.39 d (1.56) | 111.9, d | 3`` | 0.95 t (7.56) | 8.8, q | 2`` | 2.00 s | 21.3 |
| 4`` | ----- | 161.9, s | 7-O-Ac | 7-O-Ac | ||||
| 5`` | 6.92 (8.40) | 117.9, d | CO | ----- | 175.1, s | CO | ----- | 170.8, s |
| 6`` | 6.80 t (9.18) | 136.1, d | 2``` | 1.94 s | 21.5, q | 2``` | 2.10 s | 21.4 |
| 7`` | 7.58 dd (1.68, 7.98) | 119.2, d | 17-O-Nic | 17-O-Nic | ||||
| 7-O-Ac | CO | ----- | 164.9, s | CO | ----- | 165.7, s | ||
| CO | ----- | 170.8, s | 2```` | ----- | 129.7, s | 2```` | ----- | 129.7, s |
| 2``` | 2.11 s | 21.4, q | 3```` | 9.22 s | 128.4, d | 3```` | 9.15 s | 128.3, d |
| 13-O-Ac | 8.30 d (7.44) | 137.4, d | 4```` | 8.21 d (7.50) | 137.1, d | |||
| CO | ----- | 170.8, s | 7.37 m | 123.9, d | 5```` | 7.48 m | 123.9, d | |
| 2```` | 2.11 s | 21.4, q | 7.67 d (7.74) | 153.7, d | 6```` | 7.68 d (7.44) | 150.4, d | |
| 17-O-Ac | 4```` | |||||||
| CO | ----- | 170.0, s | 5```` | |||||
| 2````` | 2.12 s | 20.8, q | 6```` | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshamy, A.I.; Mohamed, T.A.; Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Dar, B.A.; Shahat, A.A.; Hegazy, M.-E.F. Euphosantianane E–G: Three New Premyrsinane Type Diterpenoids from Euphorbia sanctae-catharinae with Contribution to Chemotaxonomy. Molecules 2019, 24, 2412. https://doi.org/10.3390/molecules24132412
Elshamy AI, Mohamed TA, Al-Rowaily SL, Abd-ElGawad AM, Dar BA, Shahat AA, Hegazy M-EF. Euphosantianane E–G: Three New Premyrsinane Type Diterpenoids from Euphorbia sanctae-catharinae with Contribution to Chemotaxonomy. Molecules. 2019; 24(13):2412. https://doi.org/10.3390/molecules24132412
Chicago/Turabian StyleElshamy, Abdelsamed I., Tarik A. Mohamed, Saud L. Al-Rowaily, Ahmed M. Abd-ElGawad, Basharat A. Dar, Abdelaaty A. Shahat, and Mohamed-Elamir F. Hegazy. 2019. "Euphosantianane E–G: Three New Premyrsinane Type Diterpenoids from Euphorbia sanctae-catharinae with Contribution to Chemotaxonomy" Molecules 24, no. 13: 2412. https://doi.org/10.3390/molecules24132412
APA StyleElshamy, A. I., Mohamed, T. A., Al-Rowaily, S. L., Abd-ElGawad, A. M., Dar, B. A., Shahat, A. A., & Hegazy, M.-E. F. (2019). Euphosantianane E–G: Three New Premyrsinane Type Diterpenoids from Euphorbia sanctae-catharinae with Contribution to Chemotaxonomy. Molecules, 24(13), 2412. https://doi.org/10.3390/molecules24132412