Optimisation by Design of Experiment of Benzimidazol-2-One Synthesis under Flow Conditions
Abstract
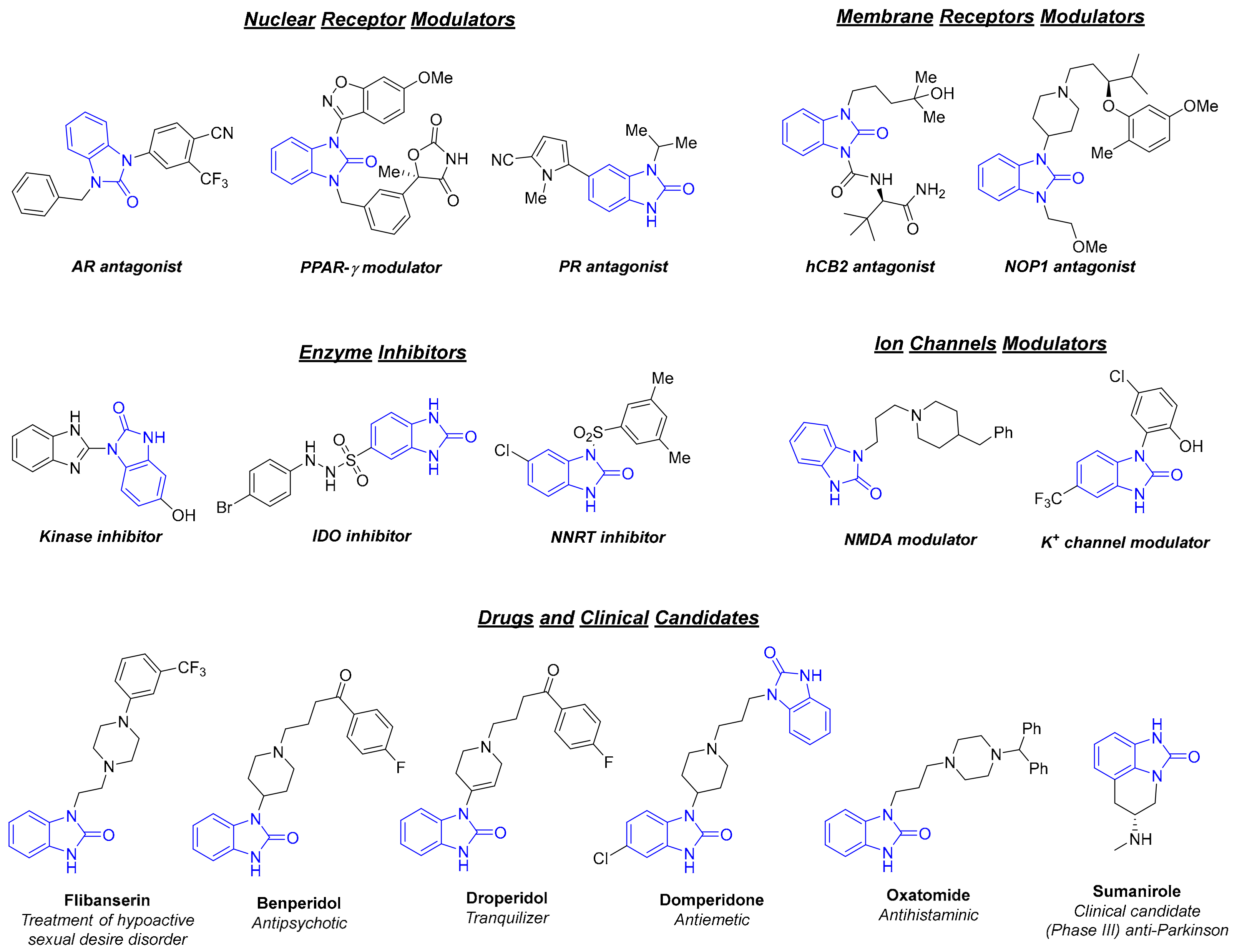
:1. Introduction
2. Results and Discussion
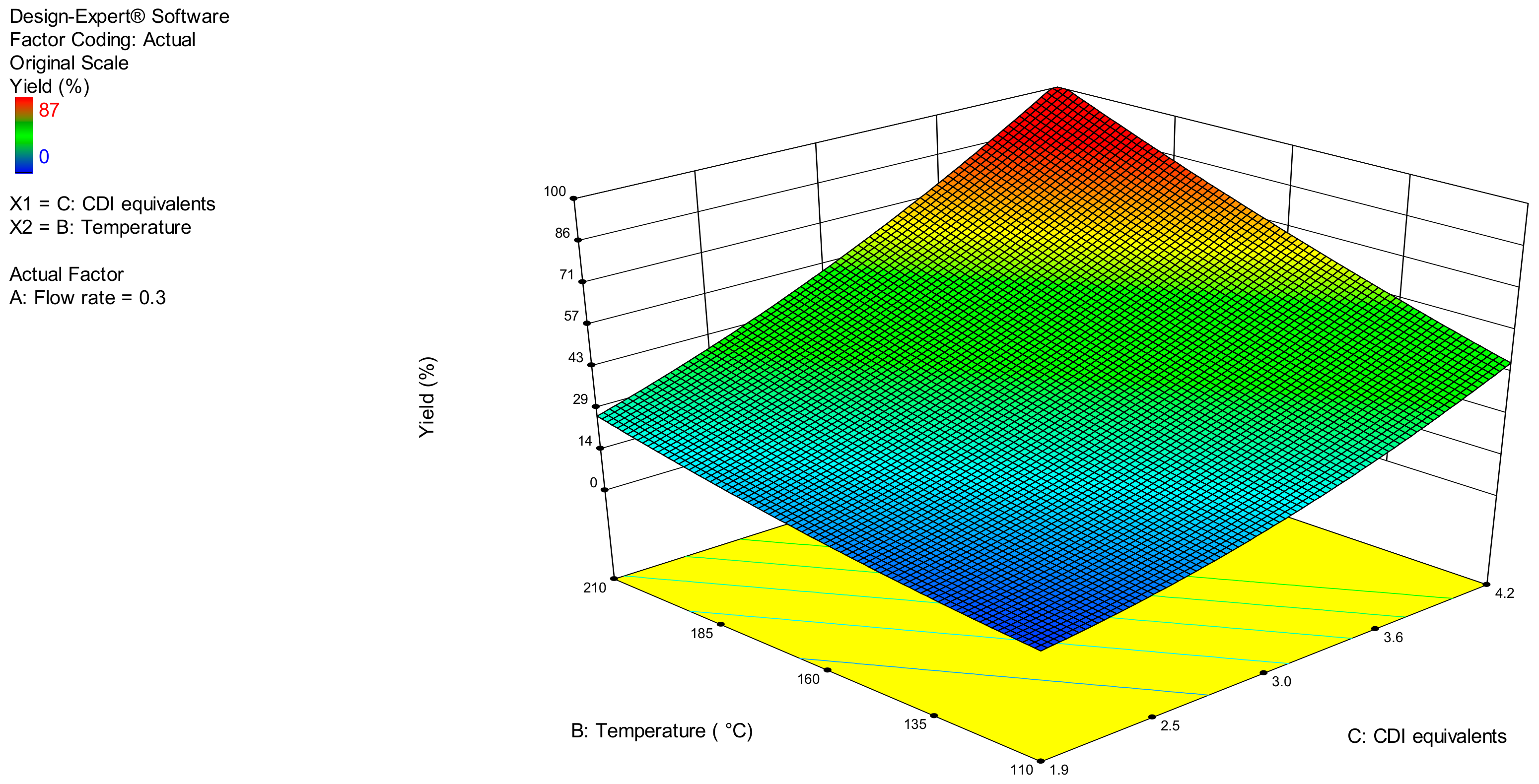
2.1. Translation under Flow Conditions and Optimisation by Design of Experiment (DoE)
2.2. Gram-Scale Flow Synthesis of N-(2-chlorobenzyl)-5-Cyano-Benzimidazol-2-One (3)
3. Material and Methods
3.1. General Methods
3.2. Protocol and Flow Set-Up for DoE Optimisation
3.3. Protocol for the Gram-Scale Flow Synthesis of N-(2-chlorobenzyl)-5-cyano-benzimidazol-2-one (3)
3.4. Compounds Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Zhukova, N.A.; Mamedov, V.A. Advances in the synthesis of benzimidazol-2-ones. Russ. Chem. Rev. 2017, 86, 968–997. [Google Scholar] [CrossRef]
- Xu, N.; Yang, C.; Gan, X.; Wei, S.; Ji, Z. Synthesis of 1-isopropyl-3-acyl-5-methyl-benzimidazolone derivatives and their antimicrobial activity. Int. J. Mol. Sci. 2013, 14, 6790–6804. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Bhatt, N.; Bhatt, P.; Joshi, H.D. Synthesis and pharmacological evaluation of novel 1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one derivatives as potential antimicrobial agents. Med. Chem. Res. 2014, 23, 2133–2139. [Google Scholar] [CrossRef]
- Monforte, A.M.; Rao, A.; Logoteta, P.; Ferro, S.; De Luca, L.; Barreca, M.L.; Iraci, N.; Maga, G.; De Clercq, E.; Pannecouque, C.; et al. Novel N1-substituted 1,3-dihydro-2H-benzimidazol-2-ones as potent non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2008, 16, 7429–7435. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lau, F.; Liu, K.; Wood, H.B.; Zhou, G.; Chen, Y.; Li, Y.; Akiyama, T.E.; Castriota, G.; Einstein, M.; et al. Benzimidazolones: A new class of selective peroxisome proliferator-activated receptor γ (PPARγ) modulators. J. Med. Chem. 2011, 54, 8541–8554. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.D.; Vinson, A.; Zhong, D.; Berrang, B.D.; Catanzaro, J.L.; Thomas, J.B.; Navarro, H.A.; Gilmour, B.P.; Deschamps, J.; Carroll, F.I. A new synthesis of the ORL-1 antagonist 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidinyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397) and activity in a calcium mobilization assay. Bioorg. Med. Chem. 2008, 16, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pandit, B.; Chettiar, S.N.; Etter, J.P.; Lewis, A.; Johnsamuel, J.; Li, P.K. Design, synthesis and biological studies of novel tubulin inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, V.A.; Zhukova, N.A.; Sinyashin, O.G. Advances in the synthesis of benzimidazolones via rearrangements of benzodiazepinones and quinoxalin(on)es. Mendeleev Commun. 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Dhanuka, I.; Simon, J.A. Flibanserin for the treatment of hypoactive sexual desire disorder in premenopausal women. Expert Opin. Pharm. 2015, 16, 2523–2529. [Google Scholar] [CrossRef]
- Kennis, L.E.J.; Vandenberk, J.; Boey, J.M.; Mertens, J.C.; Van Heertum, A.H.M.; Janssen, M.; Awouters, F. The chemical development of selective and specific serotonin S2-antagonists. Drug Dev. Res. 1986, 8, 133–140. [Google Scholar] [CrossRef]
- Richards, J.R.; Richards, I.N.; Ozery, G.; Derlet, R.W. Droperidol analgesia for opioid-tolerant patients. J. Emerg. Med. 2011, 41, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Fragen, R.J.; Caldwell, N. A new benzimidazole antiemetic, domperidone, for the treatment of postoperative nausea and vomiting. Anesthesiology 1978, 49, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.M.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Oxotamide: A review of its pharmacodynamic properties and therapeutic efficacy. Drugs 1984, 27, 210–231. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.B.; Lookingland, K.J.; Bédard, P.J.; Huff, R.M. Sumanirole, a highly dopamine D2-selective receptor agonist: In vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson’s disease. J. Pharm. Exp. Ther. 2005, 314, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.L.; Li, B.; Li, C.H.; Wang, X.; Zhang, T.Y.; Jiang, S. Preparation and enhanced visible light catalytic activity of TiO2 sensitized with benzimidazolone yellow H3G. Dye. Pigment. 2013, 98, 358–366. [Google Scholar] [CrossRef]
- Feng, J.; Xu, Y.; Sun, Y.; Wen, S.; Lei, Y.; Zhang, L.; Huo, J. Preparation and characterization of poly (ether ether ketone) s containing benzimidazolone units. J. Polym. Res. 2016, 23, 247–254. [Google Scholar] [CrossRef]
- Rudolph, C. Ueber einige Derivate des-Orthonitranilins. Ber. Dtsch. Chem. Ges. 1879, 12, 1295–1297. [Google Scholar] [CrossRef]
- Armenta, R.; Sarmiento-Sánchez, J.I. Synthesis of 1,3-dihydro-2H-benzimidazol-2-ones (microreview). Chem. Het. Compd. 2016, 52, 1002–1004. [Google Scholar] [CrossRef]
- Casnati, A.; Motti, E.; Mancuso, R.; Gabriele, B.; Della Ca’, N. Recent advances in the catalytic synthesis of imidazolidin-2-ones and benzimidazolidin-2-ones. Catalysts 2019, 9, 28. [Google Scholar] [CrossRef]
- Bana, P.; Szigetvàri, à.; Kòti, J.; Greiner, I. Flow-oriented synthetic design in the continuous preparation of the aryl piperazine drug flibanserin. React. Chem. Eng. 2019, 4, 652–657. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893, and references cited herein. [Google Scholar] [CrossRef] [PubMed]
- Banoglu, E.; Çalişkan, B.; Luderer, S.; Eren, G.; Özkan, Y.; Altenhofen, W.; Weinigel, C.; Barz, D.; Gerstmeier, J.; Pergola, C.; et al. Identification of novel benzimidazole derivatives as inhibitors of leukotriene biosynthesis by virtual screening targeting 5-lipoxygenase-activating protein (FLAP). Bioorg. Med. Chem. 2012, 20, 3728–3741. [Google Scholar] [CrossRef] [PubMed]
- Pergola, C.; Gerstmeier, J.; Mönch, B.; Çalişkan, B.; Luderer, S.; Weinigel, C.; Barz, D.; Maczewsky, J.; Pace, S.; Rossi, A.; et al. The novel benzimidazole derivative BRP-7 inhibits leukotriene biosynthesis in vitro and in vivo by targeting 5-lipoxygenase-activating protein (FLAP). Br. J. Pharmacol. 2014, 171, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Sardella, R.; Levent, S.; Ianni, F.; Çalişkan, B.; Gerstmeier, J.; Pergola, C.; Werz, O.; Banoglu, E.; Natalini, B. Chromatographic separation and biological evaluation of benzimidazole derivative enantiomers as inhibitors of leukotriene biosynthesis. J. Pharm. Biomed. Anal. 2014, 89, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Levent, S.; Gerstmeier, J.; Olgaç, A.; Nikels, F.; Garscha, U.; Carotti, A.; Macchiarulo, A.; Werz, O.; Banoglu, E.; Çalişkan, B. Synthesis and biological evaluation of C (5)-substituted derivatives of leukotriene biosynthesis inhibitor BRP-7. Eur. J. Med. Chem. 2016, 122, 510–519. [Google Scholar] [CrossRef]
- Gür, Z.T.; Çalışkan, B.; Garscha, U.; Olgaç, A.; Schubert, U.S.; Gerstmeier, J.; Werz, O.; Banoglu, E. Identification of multi-target inhibitors of leukotriene and prostaglandin E2 biosynthesis by structural tuning of the FLAP inhibitor BRP-7. Eur. J. Med. Chem. 2018, 150, 876–899. [Google Scholar] [CrossRef] [PubMed]
- Cerra, B.; Carotti, A.; Passeri, D.; Sardella, R.; Moroni, G.; Di Michele, A.; Macchiarulo, A.; Pellicciari, R.; Gioiello, A. Exploiting chemical toolboxes for the expedited generation of tetracyclic quinolines as a novel class of PXR agonists. ACS Med. Chem. Lett. 2019, 10, 677–681. [Google Scholar] [CrossRef]
- Mostarda, S.; Passeri, D.; Carotti, A.; Cerra, B.; Colliva, C.; Benicchi, T.; Macchiarulo, A.; Pellicciari, R.; Gioiello, A. Synthesis, physicochemical properties, and biological activity of bile acids 3-glucuronides: Novel insights into bile acid signalling and detoxification. Eur. J. Med. Chem. 2018, 144, 349–358. [Google Scholar] [CrossRef]
- Cerra, B.; Mostarda, S.; Custodi, C.; Macchiarulo, A.; Gioiello, A. Integrating multicomponent flow synthesis and computational approaches for the generation of a tetrahydroquinoline compound based library. Med. Chem. Commun. 2016, 7, 439–446. [Google Scholar] [CrossRef]
- Gioiello, A.; Rosatelli, E.; Teofrasti, M.; Filipponi, P.; Pellicciari, R. Building a sulfonamide library by eco-friendly flow synthesis. ACS Comb. Sci. 2013, 15, 235–239. [Google Scholar] [CrossRef]
- Gioiello, A.; Mancino, V.; Filipponi, P.; Mostarda, S.; Cerra, B. Concepts and optimization strategies of experimental design in continuous flow processing. J. Flow. Chem. 2016, 6, 167–180. [Google Scholar] [CrossRef]
- Gür, Z.T.; Çalışkan, B.; Banoglu, E. Drug discovery approaches targeting 5-lipoxygenase-activating protein (FLAP) for inhibition of cellular leukotriene biosynthesis. Eur. J. Med. Chem. 2018, 153, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, S.; Deng, Y. Important green chemistry and catalysis: Non-phosgene syntheses of isocyanates—Thermal cracking way. Chin. J. Chem. 2017, 35, 821–835. [Google Scholar] [CrossRef]
- Lanzillotto, M.; Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Mechanochemical 1,1′-carbonyldiimidazole-mediated synthesis of carbamates. ACS Sustain. Chem. Eng. 2015, 3, 2882–2889. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green. Chem. 2005, 7, 64–82. [Google Scholar]
- Butlin, R.J.; Nowak, T.; Burrows, J.N.; Block, M.H. Use of Compounds for Elevation of Pyruvate Dehydrogenase Activity. Patent WO1999062506A1, 25 June 1999. [Google Scholar]
- The cyclocarbonylation reaction of compound 4 for the gram-scale flow synthesis of N-(2-chlorobenzyl)-5-cyano-benzimidazol-2-one (3) was performed at 0.15 mL min−1 of total flow rate (τ = 67 min) instead of 0.3 mL min−1 (τ = 33 min) due to the deactivating effect of cyano group and to the sterical hindrance of 2-chlorobenzyl substituent compared to the unsubstituted o-phenylenediamine 2.
- Dekhanea, D.V.; Pawara, S.S.; Guptaa, S.V.; Shingareb, M.S.; Thore, S.N. Synthesis of benzimidazolones, benzooxazolones, 2-amino-benzothiazoles from ethyl cyanoformate and o-phenylene diamines, o-aminophenols, o-aminothiophenols promoted by lithium bromide. Lett. Org. Chem. 2011, 8, 406–411. [Google Scholar] [CrossRef]


| Variable Name | Variable Units | Range |
|---|---|---|
| Total flow rate (A) | mL min−1 | 0.10–1.00 |
| Temperature (B) | °C | 110–210 |
| CDI stoichiometry (C) | equivalents | 1.1–5.0 |
| Run | Type | Factor A (mL min−1) | Factor B (°C) | Factor C (equiv.) | Yield b |
|---|---|---|---|---|---|
| 1 | Factorial | 0.3 | 190 | 1.9 | 10 |
| 2 | Factorial | 0.8 | 190 | 1.9 | 3 |
| 3 | Axial | 1.0 | 160 | 3.0 | 8 |
| 4 | Center | 0.6 | 160 | 3.0 | 32 |
| 5 | Center | 0.6 | 160 | 3.0 | 27 |
| 6 | Axial | 0.6 | 160 | 1.1 | 0 |
| 7 | Center | 0.6 | 160 | 3.0 | 27 |
| 8 | Factorial | 0.3 | 130 | 1.9 | 0 |
| 9 | Factorial | 0.8 | 190 | 4.2 | 61 |
| 10 | Factorial | 0.8 | 130 | 4.2 | 13 |
| 11 | Factorial | 0.3 | 130 | 4.2 | 78 |
| 12 | Axial | 0.6 | 110 | 3.0 | 6 |
| 13 | Factorial | 0.8 | 130 | 1.9 | 0 |
| 14 | Factorial | 0.3 | 190 | 4.2 | 84 |
| 15 | Center | 0.6 | 160 | 3.0 | 32 |
| 16 | Axial | 0.1 | 160 | 3.0 | 87 |
| 17 | Axial | 0.6 | 210 | 3.0 | 38 |
| 18 | Axial | 0.6 | 160 | 5.0 | 38 |
| 19 | Center | 0.6 | 160 | 3.0 | 25 |
| Source | Sum of Squares | Mean Square | F Value | p-Value Prob > F a | βi b | Std. error | 95% CI Low | 95% CI High |
|---|---|---|---|---|---|---|---|---|
| Model | 129.41 | 43.14 | 22.60 | <0.0001 signif. | - | |||
| Intercept | - | 4.58 | 0.32 | 3.90 | 5.25 | |||
| A | 25.57 | 25.57 | 13.40 | 0.0023 | −1.37 | 0.37 | −2.17 | −0.57 |
| B | 14.42 | 14.42 | 7.56 | 0.0149 | 1.03 | 0.37 | 0.23 | 1.82 |
| C | 89.42 | 89.42 | 46.86 | <0.0001 | 2.56 | 0.37 | 1.76 | 3.36 |
| Lack of Fit c | 26.23 | 2.38 | 3.98 | 0.0969 non-signif. | - | |||
| Std. Dev. = 1.38 Mean = 4.58 C.V. % = 30.17 PRESS = 50.88 | R2 = 0.9827 Adj. R2 = 0.9791 d Pred. R2 = 0.9602 d Adeq. Prec. = 15.634 e | |||||||
| Optimisation Criteria | A (mL min−1) | B (°C) | C (CDI equiv.) | Predicted Yield (%) | Experimental Yield (%) b |
|---|---|---|---|---|---|
| Maximise X A, B, and C in range | 0.3 | 210 | 4.2 | 99 | 98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostarda, S.; Gür Maz, T.; Piccinno, A.; Cerra, B.; Banoglu, E. Optimisation by Design of Experiment of Benzimidazol-2-One Synthesis under Flow Conditions. Molecules 2019, 24, 2447. https://doi.org/10.3390/molecules24132447
Mostarda S, Gür Maz T, Piccinno A, Cerra B, Banoglu E. Optimisation by Design of Experiment of Benzimidazol-2-One Synthesis under Flow Conditions. Molecules. 2019; 24(13):2447. https://doi.org/10.3390/molecules24132447
Chicago/Turabian StyleMostarda, Serena, Tugçe Gür Maz, Alessandro Piccinno, Bruno Cerra, and Erden Banoglu. 2019. "Optimisation by Design of Experiment of Benzimidazol-2-One Synthesis under Flow Conditions" Molecules 24, no. 13: 2447. https://doi.org/10.3390/molecules24132447







